The Invisible Gorilla: A Classic Experiment in Perception


The invisible gorilla experiment
A couple of paragraphs above, we gave you the same instructions that Chabris and Simons gave to a group of student volunteers before doing the experiment.
When the participants finished watching the video, they were asked the following questions (answer them as well if you watched the video):
- “Did you notice anything unusual while counting the passes?”
- “Did you notice anything else besides the players?
- “Or did you notice anyone other than the players?”
- “Did you notice a gorilla?”
The last question was the one that surprised the volunteers of the invisible gorilla experiment the most. At least 58% of them. Whenever the experiment has been repeated, the percentage of surprise is more or less the same. Yes, there was a gorilla in the video, but more than half of the people didn’t notice it. Did you see it?
The reactions to what happened
The first time the invisible gorilla experiment was conducted, and all subsequent ones, most of those who participated and didn’t notice the presence of the gorilla were amazed at how clear it all was! It seemed impossible to them that they had overlooked something so obvious.
When they’re asked to watch the video again, they all see the gorilla without a problem. Some think that they’ve been shown two different videos, but, of course, this isn’t the case. This experiment won the Ig Nobel Prize. This is an award given to those scientific activities that “first make you laugh and then make you think”.
Why are so many people blind to such an obvious image in the video? That’s the big question that comes out of this. It’s also striking that so many people refuse to accept that their eyes and perception are deceiving them. They think they’re seeing everything correctly, and yet they haven’t seen something so obvious.

Broadbent’s Filter Model
Broadbent’s Attentional Theory, also known as the Filter Theory of Attention, proposes that humans can only process a limited amount of sensory information at any given time due to an attentional “bottleneck.”
Broadbent (1958) proposed that the physical characteristics of messages are used to select one message for further processing and that all others are lost.
Information from all stimuli presented at any time enters an unlimited-capacity sensory buffer.
One of the inputs is then selected based on its physical characteristics (such as pitch or loudness) for further processing by being allowed to pass through a filter.
Because we have only a limited capacity to process information, this filter is designed to prevent the information-processing system from becoming overloaded.
The inputs not initially selected by the filter remain briefly in the sensory buffer store, and if they are not processed, they decay rapidly. Broadbent assumed that the filter rejected the unattended message at an early processing stage.
According to Broadbent, the meaning of any of the messages is not taken into account at all by the filter. All semantic processing is carried out after the filter has selected the message to pay attention to. So whichever message(s) are restricted by the bottleneck (i.e., not selective) is not understood.
Broadbent wanted to see how people could focus their attention (selectively attend), and to do this; he deliberately overloaded them with stimuli.
One of the ways Broadbent achieved this was by simultaneously sending one message to a person’s right ear and a different message to their left ear.
This is called a split-span experiment (the dichotic listening task).
Dichotic Listening Task
The dichotic listening tasks involves simultaneously sending one message (a 3-digit number) to a person’s right ear and a different message (a different 3-digit number) to their left ear.
Participants were asked to listen to both messages simultaneously and repeat what they heard. This is known as a “dichotic listening task.”
Broadbent was interested in how these would be repeated back. Would the participant repeat the digits back in the order that they were heard (order of presentation), or repeat back what was heard in one ear followed by the other ear (ear-by-ear).
He found that people made fewer mistakes repeating back ear by ear and would usually repeat back this way.
Evaluation of Broadbent’s Model
1. Broadbent’s dichotic listening experiments have been criticized because:
- The early studies all used people who were unfamiliar with shadowing and so found it very difficult and demanding. Eysenck and Keane (1990) claim that the inability of naive participants to shadow successfully is due to their unfamiliarity with the shadowing task rather than an inability of the attentional system.
- Participants reported after the entire message had been played – it is possible that the unattended message is analyzed thoroughly, but participants forget.
- Analysis of the unattended message might occur below the level of conscious awareness. For example, research by Von Wright et al. (1975) indicated analysis of the unattended message in a shadowing task. A word was first presented to participants with a mild electric shock. When the same word was later presented to the unattended channel, participants registered an increase in GSR (indicative of emotional arousal and analysis of the word in the unattended channel).
- More recent research has indicated the above points are important: e.g., Moray (1959) studied the effects of the practice. Naive subjects could only detect 8% of digits appearing in either the shadowed or non-shadowed message; Moray (an experienced “shadower”) detected 67%.
2. Broadbent’s theory predicts that hearing your name when you are not paying attention should be impossible because unattended messages are filtered out before you process the meaning – thus, the model cannot account for the “Cocktail Party Phenomenon.”
3 . Other researchers have demonstrated the “ cocktail party effect ” (Cherry, 1953) under experimental conditions and have discovered occasions when information heard in the unattended ear “broke through” to interfere with information participants are paying attention to in the other ear.
This implies some analysis of the meaning of stimuli must have occurred prior to the selection of channels. In Broadbent’s model, the filter is based solely on sensory analysis of the physical characteristics of the stimuli.
Treisman’s Attenuation Model
Treisman (1964) agrees with Broadbent’s theory of an early bottleneck filter. However, the difference is that Treisman’s filter attenuates rather than eliminates the unattended material.
Attenuation is like turning down the volume so that if you have four sources of sound in one room (TV, radio, people talking, baby crying), you can turn down or attenuate 3 to attend to the fourth.
This means people can still process the meaning of the attended message(s).
In her experiments, Treisman demonstrated that participants could still identify the contents of an unattended message, indicating that they were able to process the meaning of both the attended and unattended messages.
Treisman carried out dichotic listening tasks using the speech shadowing method. Typically, in this method, participants are asked to simultaneously repeat aloud speech played into one ear (called the attended ear) while another message is spoken to the other ear.
For example, participants were asked to shadow “I saw the girl furniture over” and ignore “me that bird green jumping fee,” reported hearing “I saw the girl jumping over.”
Clearly, then, the unattended message was being processed for meaning, and Broadbent’s Filter Model, where the filter was extracted based on physical characteristics only, could not explain these findings. The evidence suggests that Broadbent’s Filter Model is inadequate and does not allow for meaning to be taken into account.
Evaluation of Treisman’s Model
1. Treisman’s Model overcomes some of the problems associated with Broadbent’s Filter Model, e.g., the Attenuation Model can account for the “Cocktail Party Syndrome.”
2. Treisman’s model does not explain how exactly semantic analysis works.
3. The nature of the attenuation process has never been precisely specified.
4. A problem with all dichotic listening experiments is that you can never be sure that the participants have not actually switched attention to the so-called unattended channel.
Broadbent, D. (1958). Perception and Communication. London: Pergamon Press.
Cherry, E. C. (1953). Some experiments on the recognition of speech with one and with two ears. Journal of the Acoustical Society of America , 25, 975–979.
Eysenck, M. W. & Keane, M. T. (1990). Cognitive psychology: a student’s handbook . Hove: Lawrence Erlbaum Associates Ltd.
Moray, N. P. (1959). Attention in dichotic listening: Affective cues and the influence of instructions. Quarterly Journal of Experimental Psychology , 11, 56–60.
Treisman, A., 1964. Selective attention in man. British Medical Bulletin , 20, 12-16.
Von Wright, J. M., Anderson, K., & Stenman, U. (1975). Generalization of conditioned GSRs in dichotic listening. In P. M. A. Rabbitt & S. Dornic (Eds.), Attention and performance (Vol. V, pp. 194–204). London: Academic Press.
Keep Learning
How We Use Selective Attention to Filter Information and Focus
BBC Radio: Donald Broadbent and the Cocktail Party.
Attention Journal Article
Attention Essay

An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Working Memory and Attention – A Conceptual Analysis and Review
Klaus oberauer.
- Author information
- Article notes
- Copyright and License information
Received 2018 Dec 2; Accepted 2019 Feb 14; Collection date 2019.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC-BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. See http://creativecommons.org/licenses/by/4.0/ .
There is broad agreement that working memory is closely related to attention. This article delineates several theoretical options for conceptualizing this link, and evaluates their viability in light of their theoretical implications and the empirical support they received. A first divide exists between the concept of attention as a limited resource, and the concept of attention as selective information processing. Theories conceptualizing attention as a resource assume that this resource is responsible for the limited capacity of working memory. Three versions of this idea have been proposed: Attention as a resource for storage and processing, a shared resource for perceptual attention and memory maintenance, and a resource for the control of attention. The first of these three is empirically well supported, but the other two are not. By contrast, when attention is understood as a selection mechanism, it is usually not invoked to explain the capacity limit of working memory – rather, researchers ask how different forms of attention interact with working memory, in two areas. The first pertains to attentional selection of the contents of working memory, controlled by mechanisms of filtering out irrelevant stimuli, and removing no-longer relevant representations from working memory. Within working memory contents, a single item is often selected into the focus of attention for processing. The second area pertains to the role of working memory in cognitive control. Working memory contributes to controlling perceptual attention – by holding templates for targets of perceptual selection – and controlling action – by holding task sets to implement our current goals.
Keywords: Working memory, Attention, Cognitive Control
There is a broad consensus that working memory and attention are intimately linked ( Awh, Jonides, & Reuter-Lorenz, 1998 ; Baddeley, 1993 ; Chun, 2011 ; Cowan, 1995 ; Gazzaley & Nobre, 2012 ; Kane, Bleckley, Conway, & Engle, 2001 ; Kiyonaga & Egner, 2014 ; Oberauer, 2009 ; Olivers, 2008 ). But what is it that we agree upon? Both working memory and attention can be conceptualized in different ways, resulting in a broad array of theoretical options for linking them. The purpose of this review is to propose a map for organizing these theoretical options, delineate their implications, and to evaluate the evidence for each of them.
The meaning of the concept working memory (WM) depends on the theory in which the concept figures. The definitions reviewed by Cowan ( 2017 ) differ primarily in the substantive assumptions they include (e.g., whether or not WM consists of multiple storage modules, and to what extent it includes long-term memory). Beyond these differences in theoretical assumptions, however, there is a broad consensus on what the term working memory refers to: The mechanisms and processes that hold the mental representations currently most needed for an ongoing cognitive task available for processing.
The meanings of the term attention are more diverse, as they reflect distinctions not only of definitions but also of different referents of the term: Attention is not a unitary entity ( Chun, Golomb, & Turk-Browne, 2011 ). Conceptualizations of attention can be distinguished along several dimensions that provide a coordinate system for our conceptual map. A first distinction pertains to how attention is defined. One definition of attention characterizes it as a limited resource for information processing (e.g., Wickens, 1980 ). Another concept of attention is as a process of (or mechanism for) selection of information to be processed with priority (e.g., Chun et al., 2011 ; Desimone & Duncan, 1995 ). These two concepts of attention play different roles in theorizing about working memory, and I will discuss them in turn below.
A second distinction pertains to what we attend to. I find it useful to distinguish the possible objects of attention along two dimensions (see Table 1 ). 1 First, we can distinguish between attention to our currently perceived environment (e.g., attention to visual objects or auditory streams) from attention to information currently not perceived, such as attention to remembered episodes or concepts that we think about. 2 Second, we can distinguish between attention to things and events in the world around us on the one hand, and attention to our own goals and (mental or overt) actions on the other. The latter form of attention includes selection of our current goal or task set and shielding it from distraction ( Kane & Engle, 2003 ; Monsell, 2003 ), selection of one of several possible actions ( Pashler, 1994 ), and monitoring of our actions and their outcomes ( Yeung, Botvinick, & Cohen, 2004 ).
A Taxonomy of Attention.
Note: Descriptions pertaining to attention as selection/prioritization are printed in regular font; descriptions pertaining to attention as a resource in italics.
A third distinction pertains to the forces that determine what we attend to – this is the distinction between controlled and automatic deployment of attention ( Shiffrin & Schneider, 1977 ). Attention is controlled when it is directed according to our current goals. The influence of current goals on attention is often referred to as “top-down”. Attention is automatic to the extent that its direction is influenced by forces independent of our current goals – these include the “bottom-up” attraction of attention by perceived properties of the stimuli (e.g., their “salience”) as well as influences of our learning history on what we attend to, for instance when attention is drawn to information that we have learned to be relevant ( Awh, Belopolsky, & Theeuwes, 2012 ; Theeuwes, 2018 ).
The concept of executive attention is often used when discussing the relation between attention and working memory. Executive attention is a term that is notoriously poorly defined ( Jurado & Rosselli, 2007 ). It is used on the one hand to refer to attention directed to one’s own goals and (mental or overt) actions, including response selection ( Szmalec, Vandierendonck, & Kemps, 2005 ), action planning, protecting the pursuit of our current goal from distractions and temptations, as well as switching from one task to another. On the other hand, executive attention is also used to refer to the top-down control of attention, including attention to things and events in the environment – for keeping our attention on the relevant stimuli or features and avoiding distraction by irrelevant ones, as in the Stroop task and the flanker task. As such, the term executive attention is used to denote one pole on each of two dimensions in my proposed taxonomy, one pertaining to the objects of attention (things and events in the world vs. our own goals and actions), the other pertaining to what determines the orientation of attention (controlled vs. automatic). The first meaning assigns executive attention a function in controlling our thoughts and actions (including what we attend to) whereas the second states that executive attention is itself controlled. One way to perhaps bring together the two meanings is by assuming that we attend to (i.e., select, assign resources to) our own goals and actions – including the action of attending to some object – in order to control them. Nevertheless, I find the term executive attention disquietingly ambiguous, and therefore will use instead the terms attention to (cognitive) action and controlled attention to refer to the two aspects of executive attention, respectively.
I organize the review by the two definitions of attention – as a resource or as a selection mechanism – because they have different implications for how attention and working memory are related. Within each section I will discuss the different objects of attention, and the different modes of control.
Attention as a Resource
The idea of attention as a resource is that the cognitive system has a limited resource that can be used for carrying out so-called attention-demanding processes. The resource is assumed to be a continuous quantity that can be split arbitrarily and allotted to different processes, depending on task demands. Processing efficiency (i.e., speed, accuracy) is a positive monotonic function of the amount of resource assigned to a process ( Navon & Gopher, 1979 ). The assumption that WM capacity reflects a limited resource has a long tradition ( Anderson, Reder, & Lebiere, 1996 ; Case, 1972 ; Just & Carpenter, 1980 ; Ma, Husain, & Bays, 2014 ). Authors linking WM to an attentional resource are endorsing the view that the limited capacity of WM reflects a limited resource, and that this resource also serves some (or all) functions commonly ascribed to attention. Three versions of this idea can be distinguished by which functions the attentional resource is assumed to be needed for: (1) storage and processing of information (e.g., Just & Carpenter, 1992 ), (2) perceptual attention and memory maintenance (e.g., Ester, Fukuda, May, Vogel, & Awh, 2014 ; Kiyonaga & Egner, 2014 ), or (3) the control of attention (e.g., Allen, Baddeley, & Hitch, 2006 ; Baddeley, 1993 , 1996 ; Lavie, 2005 ).
Attention for Storage and Processing
Many theorists discussing the relation between working memory and attention characterize attention as a limited resource for maintaining representations in an “active”, available state ( Cowan, 2005 ). Often this resource is assumed to be shared between “storage” and “processing” ( Case, Kurland, & Goldberg, 1982 ; Cowan et al., 2005 ; Just & Carpenter, 1992 ). According to this view, the same attentional resource is required for keeping representations available and for carrying out certain basic cognitive processes such as selecting a response to a stimulus. A prediction from this theory is that attention-demanding cognitive processes compete with concurrent storage ( Z. Chen & Cowan, 2009 ).
There are two variants of this theoretical idea. One is that a share of the resource needs to be continuously assigned to a representation to keep it in WM ( Case et al., 1982 ). The other is that attention is required directly only for processing, not storage. In this view attention indirectly contributes to memory maintenance because it is needed for refreshing WM representations, which would otherwise decay ( Barrouillet, Bernardin, & Camos, 2004 ). Barrouillet and colleagues further specify the resource required for refreshing as the limited resource for so-called central processes, such as response selection ( Barrouillet, Bernardin, Portrat, Vergauwe, & Camos, 2007 ). Dual-task studies with variants of the PRP (psychological refractory period) paradigm have established a strong capacity limit on central processes ( Pashler, 1994 ), which has been explained by a limited central-attentional resource ( Navon & Miller, 2002 ; Tombu & Jolicoeur, 2003 ).
Theorists linking WM to attention as resource commonly assume that there is a single, content-general attentional resource. It follows that storage and processing compete with each other whether or not they share any contents. This assumption leads to the prediction of dual-task costs when WM storage and processing demands from very different contents are combined with each other. There is considerable evidence confirming this prediction ( Chein, Moore, & Conway, 2011 ; Morey & Bieler, 2012 ; Saults & Cowan, 2007 ; Vergauwe, Barrouillet, & Camos, 2010 ), lending support to the notion that WM capacity is limited by an attentional resource. There is also evidence that storage and processing compete for central processing capacity: The extent to which maintenance in WM is impaired by concurrent processing is a monotonic function of cognitive load , defined as the proportion of time during which central attention is engaged by the processing demand ( Barrouillet et al., 2007 ).
One problem for the assumption of a shared resource for storage and processing is that, although a memory load reduces the efficiency of concurrent response-selection tasks, that dual-task cost diminishes substantially over the first few seconds of the retention interval ( Jolicoeur & Dell’Acqua, 1998 ; Thalmann, Souza, & Oberauer, 2019 ; Vergauwe, Camos, & Barrouillet, 2014 ), and is often not observed at all when there is an unfilled interval of a few seconds between encoding of the memory set and commencement of the processing task ( Hazeltine & Witfall, 2011 ; Klapp, Marshburn, & Lester, 1983 ; Oberauer, Demmrich, Mayr, & Kliegl, 2001 ). This observation has already led Klapp and colleagues ( 1983 ) to question the idea of a shared resource for storage and processing: To uphold this idea we would have to assume that the resource demand of maintenance dwindles to a negligible level within a few seconds. This would be compatible with the assumption that a central processing resource is required for short-term consolidation of information in working memory ( Jolicoeur & Dell’Acqua, 1998 ; Nieuwenstein & Wyble, 2014 ; Ricker & Hardman, 2017 ) but not with the assumption that a resource is needed for maintenance throughout the retention interval.
As mentioned above, the assumption of shared resources for storage and processing comes in two variants: The first, traditional one is that a representation needs a share of the resource assigned to it to be in WM, and the same resource is needed for carrying out cognitive operations. The second variant is that maintenance processing such as refreshing share a limited resource with other cognitive operations ( Barrouillet et al., 2004 ). The second variant rests on the premise that without refreshing the representations in WM decay – only on that assumption does the processing resource assigned to refreshing become essential for WM maintenance. The decay assumption, however, is probably not true, at least for verbal materials ( Oberauer & Lewandowsky, 2013 , 2014 ).
The first variant has a conceptual problem: Simultaneous maintenance and processing compete for a shared resource only until the processing task is completed – after that, the full resource can be re-assigned to the representations in WM. Why then should memory performance suffer from a concurrent processing task although memory is tested only after the processing task is done? (for a more detailed treatment see Oberauer, Farrell, Jarrold, & Lewandowsky, 2016 ). The problem is illustrated by a study that, according to the authors, reveals the neuronal basis of resource sharing: Watanabe and Funahashi ( 2014 ) recorded from multiple neurons in the lateral pre-frontal cortex (LPFC) while monkeys did a spatial attention task, a spatial WM task, or a dual-task combination of the two. The two tasks recruited largely overlapping LPFC neurons, which showed spatial selectivity when each task was done alone. While both tasks were done simultaneously, the LPFC neurons lost most of their spatial selectivity, and collectively their firing rate pattern contained less information about the attended location and the remembered location during that period. After the attention task was completed, however, the information about the location in memory was “reawakened” in the firing pattern of the LPFC neurons, reaching the same strength as in the single-task condition. The authors did observe a (small) performance decrement in the dual-task relative to the single-task condition, but that dual-task cost is not explained by their neural data – looking at the neural data, we would expect no detrimental effect on memory by the concurrent attention task.
To conclude, the assumption of a shared resource for memory retention and central processes has received much empirical support. At the same time, it is challenged by the finding that dual-task costs on processing speed tend to vanish over time, and – depending on the version endorsed – the lack of evidence for decay, and the problem of how to explain that the competition between processing and storage affects memory performance after the competition has ended.
Attention for Perception and Memory
A resource shared between “storage” and “processing” spans both sides of the distinction between attention to things and events (i.e., the information to be stored), and attention to goals and actions (i.e., to the task sets guiding the processing operations). We can also ask whether the same resource applies to both sides of another distinction, the one between perceptual attention and attention to not-perceived objects. Most task paradigms for studying WM require retention of information in the absence of perceptual input. There is evidence, however, that the limited capacity of WM applies not only to information in memory but equally to information still in view. Tsubomi, Fukuda, Watanabe, and Vogel ( 2013 ) measured the contralateral delay activity (CDA), a neural marker of the number of objects a person holds in visual WM ( Luria, Balaban, Awh, & Vogel, 2016 ; Vogel & Machizawa, 2004 ) while participants attended to a variable number of color patches still in view, or attempted to remember them after their offset. In both cases, the CDA amplitude increased with set size up to about 3 items and then levelled off. Individual CDA amplitudes correlated with performance on a test of one randomly selected item regardless of whether that item remained in view until the time of test or had to be retained in memory for a second.
The study of Tsubomi et al. ( 2013 ) shows striking similarities between the capacity limits for attending to perceptual stimuli and for maintaining stimuli in memory (see also Ester et al., 2014 ). Still, these two functions could rely on separate resources that happen to bear similarities to each other. If the same limited resource underlies perceptual attention and maintenance in WM, then demanding both at the same time should incur a substantial dual-task cost, such that when the load of one task is increased, performance on the other suffers. The evidence for this prediction is mixed. Fougnie and Marois ( 2006 ) found load-dependent dual-task costs when combining a visual WM task with a visual attention task (simultaneous tracking of multiple moving objects, or monitoring multiple parallel streams of rapidly presented visual stimuli for a target) but these costs were less than the cost of combining two visual WM tasks. Souza and Oberauer ( 2017 ) found only negligible dual-task costs when inserting a visual attention task (monitoring a stimulus for a subtle brightness change) in the retention interval of a visual WM task. Several studies investigated dual-task costs between WM and visual search. These dual-task costs increase with the load on each of the two tasks – as expected on the assumption of a shared resource – only when the contents of WM were spatial locations (for a review see Woodman & Chun, 2006 ). To conclude, although attending to perceptual information and maintaining information in WM after it disappeared from the environment have much in common, the evidence that they share a limited resource is not yet convincing.
Controlled Attention
The concept of attention as a limited resource is often linked specifically to controlled attention, whereas automatic attention is thought not to be resource demanding ( Schneider & Shiffrin, 1977 ; Shiffrin & Schneider, 1977 ). There are two ways in which this link can be spelled out: (a) Attention that is allocated in a controlled manner – according to “top down” influences from our current goals – underlies a resource limit but attention that is automatically attracted to some information independent of its relevance for our current goal does not underlie that resource limit. Stated in this way we face the awkward conclusion that allocating attention to the same object (e.g., a red traffic light in a street scene, or a word we hold in WM) does or does not rely on a limited resource depending on what forces led attention to that object. The same cognitive function – prioritizing processing of the attended information – would be resource consuming or not depending on how it was invoked.
In my view, a less awkward interpretation is: (b) Paying attention to an object does not require a resource per se – rather the process of controlling attention in a top-down manner consumes the limited resource. This interpretation reflects how Shiffrin and Schneider ( 1977, p. 156 ) explain why controlled processes are capacity limited: These processes need to be controlled by continuously paying attention to them, and attention cannot be allocated to more than one process at a time. In other words, the attentional resource imposes a bottleneck on the control processes, not on the controlled processes. The limitation is on how many different (cognitive or overt) actions we can attend to at the same time in order to control them. For instance, in visual search, perceptual attention can be drawn to some stimuli automatically, and theoretically there is no limit on how many such forces exert their pull in parallel. Perceptual attention can also be directed in a controlled manner – by attending to the action of deploying attention to visual stimuli – and this control process is limited to one action at a time. The limitation does not rest with the controlled attention – a limit on how many visual stimuli can be attended at the same time – but with the controlling attention.
This conception of an attentional resource differs from the preceding two. The notion of a resource for storage and processing and the idea of a shared attentional resource for perception and memory share the assumption that the resource is allocated to representations of objects and events that we perceive or hold in WM. In contrast, the “attentional control” idea assumes a resource for the control of what we attend to, and more generally, of what we think and do. These conceptualizations have different implications when we apply them to WM. For instance, consider a situation in which WM receives an overload of information, some of which is relevant and some of which is irrelevant. Examples of this scenario are the complex-span paradigm ( Daneman & Carpenter, 1980 ), in which to-be-remembered items alternate with stimuli to be processed but not retained, or the filtering paradigm ( Vogel, McCollough, & Machizawa, 2005 ), in which participants see an array of visual stimuli and need to remember a pre-defined subset (e.g., only the red objects). According to theories assuming a limited resource allocated to representations in WM, attention limits how much of the given information can be retained, and a separate parameter determines the filtering efficiency, that is, the extent to which the cognitive system manages to keep the distractor information out of WM, so that it does not consume part of the valuable storage resource. These theories predict that individuals with lower WM capacity maintain a smaller amount of both relevant and irrelevant information, but their proportion, reflecting filtering efficiency, should be independent of WM capacity. According to the controlled-attention view, by contrast, the attentional resource determines the filtering efficiency. Hence, individuals with lower WM capacity retain the same amount of information as those with higher capacity, but people differing in WM capacity differ in the ratio of relevant to irrelevant information that they retain.
Paradoxes lurk when we try to combine the two notions of attentional resources, assuming that the same limited resource is required for both storage and control: According to this fusion version of the attentional-resource idea, keeping some irrelevant piece of information out of WM, or removing it from WM, consumes attentional resource (because it is an act of control over what we attend to) and at the same time frees up attentional resource (because it reduces the amount of information that is held in WM). In the same manner, stopping a cognitive process costs attentional resource but at the same time frees up attentional resource. With such a conception, it becomes virtually impossible to say whether some cognitive process – such as filtering or deleting information from WM – renders a net cost or a net gain in resource. As a consequence, the theory becomes untestable. This problem needs to be kept in mind when attempts are made to reconcile the two versions of attentional-resource theories of WM (e.g., Cowan, Fristoe, Elliott, Brunner, & Saults, 2006 ). 3
If WM and the control of attention share a limited resource, we should expect substantial dual-task costs when an attention-control demand is combined with WM maintenance. Evidence for such a dual-task cost comes from studies demonstrating that a load on WM increases people’s susceptibility to distraction, for instance by the irrelevant stimuli in a flanker task ( Kelley & Lavie, 2011 ; Lavie, Hirst, de Fockert, & Viding, 2004 ). Interpretation of this result is complicated by the observation that only a verbal WM load increases the flanker effect – a visual WM load has the opposite effect ( Konstantinou, Beal, King, & Lavie, 2014 ; Konstantinou & Lavie, 2013 ). Konstantinou et al. ( 2014 ) explain this dissociation by assuming that visual WM contents place a load on a visual perceptual resource, and increasing the load on perceptual resources has been shown to reduce flanker interference ( Lavie, 2005 ). In contrast, verbal WM relies on rehearsal for maintenance, and rehearsal competes for a shared attentional-control resource with the control of visual attention. The latter assumption is at odds with the position of most other resource theorists, who assume that rehearsal requires little, if any such resource ( Baddeley, 1986 ; Camos, Lagner, & Barrouillet, 2009 ; Cowan, 2001 ). Other studies provide further evidence that a load on WM can both increase and decrease people’s distractability by a flanker stimulus during a perceptual comparison task: When the category of stimuli held in WM matched that of the targets of the comparison task (but not that of the flankers), the flanker compatibility effect increased, but when the WM contents matched the category of the flankers, and not the targets, then the flanker compatibility effect decreased under load compared to no load ( Kim, Kim, & Chun, 2005 ; Park, Kim, & Chun, 2007 ). Taken together, there is no convincing evidence that loading WM depletes a resource needed for the control of attention.
We can also ask whether concurrent demands on the control of attention impair performance in a WM task. This appears not to be the case. The effect of concurrent processing on memory is larger when the processing task requires more attention control (e.g., task switching vs. task repetition, incongruent vs. neutral Stroop trials), but that effect is entirely accounted for by the longer duration of response selection in the more difficult conditions ( Barrouillet, Portrat, & Camos, 2011 ; Liefooghe, Barrouillet, Vandierendonck, & Camos, 2008 ). Hence, the dual-task cost of concurrent processing for memory is a function of the demand on central attention for action selection, not the demand on the control of attention. Moreover, Lawrence, Myerson, Oonk, and Abrams ( 2001 ) found that when people had to make saccades to irrelevant locations during the retention interval, memory performance is impaired, in particular for spatial information. That effect was equally large for reflexive saccades towards a suddenly appearing target and for controlled anti-saccades away from a target, contrary to the assumption that the control of attention in the anti-saccade condition competes for WM resources. Bunting, Cowan, and Colflesh ( 2008 ) used a manual analog of the anti-saccade task as distractor activity during the retention interval, and found significantly worse performance in the anti-press than the pro-press condition in only 3 out of 12 experimental conditions.
A second prediction from the assumption that WM maintenance and controlled attention share a resource is that measures of the efficiency of the two should be correlated across individuals. This prediction has been tested with regard to two forms of control over the contents of WM ( Hasher, Zacks, & May, 1999 ): Filtering irrelevant stimuli at encoding so that they never enter WM, and removal of no-longer relevant stimuli from WM after they have been encoded. Support for the prediction comes from studies measuring filtering efficiency in visual change-detection tasks through the effect of irrelevant stimuli on the CDA ( Vogel et al., 2005 ). Individual differences in filtering efficiency are strongly correlated with accuracy in change detection ( Luria et al., 2016 ). However, when Mall, Morey, Wolff, and Lehnert ( 2014 ) measured filtering efficiency through behavioral indicators – the performance gain from being able to ignore half the stimuli in the array, and the proportion of time people fixated on locations of irrelevant stimuli during encoding and retention – they found no correlation with people’s WM capacity, measured through complex-span tasks. One possible interpretation is that controlled attention (as indexed by filtering) and WM maintenance share a resource that is not domain general but rather specific to visual stimuli. Removal efficiency has been measured through the speed with which people remove to-be-updated information from WM in an updating paradigm ( Ecker, Lewandowsky, & Oberauer, 2014 ). Whereas this first study showed no correlation of removal efficiency with WM capacity, a subsequent study measuring removal efficiency through a larger set of updating tasks observed a small positive correlation ( Singh, Gignac, Brydges, & Ecker, 2018 ). This result could reflect a shared resource for WM maintenance and attentional control. Alternatively, it could mean that people who efficiently remove no-longer relevant information from WM are better at reducing interference from that information in WM, which improves their ability to retrieve the relevant information ( Oberauer, Lewandowsky, Farrell, Jarrold, & Greaves, 2012 ).
Other research investigated the correlation between WM capacity and measures of attentional control outside the context of WM tasks, for instance the ability to attend to relevant and ignore irrelevant stimuli or features in perceptual decision tasks (e.g., the Stroop, flanker, or Simon task), the ability to suppress a strong action tendency (e.g., moving the eyes away from a suddenly appearing stimulus in the anti-saccade task), or the ability to stop an already prepared action (i.e., the stop-signal paradigm). Numerous studies have found positive correlations between WM capacity and these measures of attention control (e.g., Chuderski, 2014 ; McVay & Kane, 2012 ; Shipstead, Lindsey, Marshall, & Engle, 2014 ; Unsworth, 2015 ; Unsworth, Fukuda, Awh, & Vogel, 2014 ), whereas a few others failed to find such a relationship ( Keye, Wilhelm, Oberauer, & van Ravenzwaaij, 2009 ; Wilhelm, Hildebrandt, & Oberauer, 2013 ). Additional support comes from findings of a positive correlation between WM capacity and people’s self-reported mind wandering in response to thought probes during a cognitive task ( McVay & Kane, 2009 , 2012 ; Randall, Oswald, & Beier, 2014 ).
Taken together, the evidence for a close relation between WM and the control of attention is mixed. The most convincing evidence comes from correlational studies linking WM capacity to indicators of attention control from tasks without a memory demand. There is some evidence that WM capacity is also correlated with the efficiency of controlling the contents of WM through filtering and removal, but it is yet too weak and inconsistent to draw strong conclusions. This correlational evidence, however, can be explained without invoking the notion of a shared resource, as I’ll discuss below (in the section “How is WM related to the control of attention and action?”). The experimental evidence from dual-task costs speaks against competition between WM maintenance and attention control for a shared resource.
I have considered three theoretical options for spelling out the idea of WM as relying on an attentional resource: (1) a shared resource for “storage” and “processing”, (2) a shared resource for perceptual attention and WM, and (3) a shared resource for attention control and WM. Of these three, the first option has received the most convincing empirical support, but it also suffers from empirical challenges, and from the conceptual problem of explaining how the competition for resources between storage and processing can have an impact on memory performance after the competition is over. I do not see these challenges as fatal – it is probably still too early to announce the “demise” ( Klapp et al., 1983 ) of the idea that WM is limited by an attentional resource – but theorists working with this concept should aim to address these challenges. In the remainder of this article I discuss the relation of WM to attention from the perspective that attention is the selection and prioritization of information, which does not entail a commitment to a limited resource.
Attention as Selection
A different perspective on the relation between WM and attention emerges when attention is defined not as a resource but as a mechanism for selecting and prioritizing representations. In this perspective, attention does not explain the capacity limit of WM. Rather, we should consider WM as an instance of attention – specifically, WM is attention to memory representations. Holding a set of representations in WM means selecting them from among all the representations that our mind is capable of, thereby rendering them available as input for cognitive operations. As such, WM meets the definition of attention as a mechanism of selection ( Oberauer, 2009 ). In this perspective, the relationship between the concept of WM and the concept of attention is not an empirical but a conceptual one.
Nevertheless, we can ask several empirical questions about how WM is related to attention as a selection mechanism: (1) How is information selected into WM? (2) How is information selected within WM? (3) What is the relation between attention to memory and attention to perceived stimuli – are they the same, and if not, how do they influence each other? (4) How is WM related to the control of attention and action? I next address these questions in turn.
How is Information Selected into Working Memory?
Information can be selected to be brought into WM from perception or from long-term memory. This selection is to a large extent controlled: People are very good, though not perfect, at letting only relevant information into WM. Moreover, people also have control over which information to keep in WM and which to remove.
Filtering Perceptual Information. With regard to perceived information, perceptual attention arguably plays an important role in selecting which stimuli are encoded into WM. Stimuli that are known to be irrelevant from the start, and are easy to discriminate from relevant stimuli, can be filtered out very effectively ( Baddeley, Papagno, & Andrade, 1993 ), though not always perfectly ( Ueno, Allen, Baddeley, Hitch, & Saito, 2011 ; Vogel et al., 2005 ); children and older adults seem to have more difficulty with filtering irrelevant stimuli at encoding ( Sander, Werkle-Bergner, & Lindenberger, 2011 ). A question discussed in the context of visual WM is whether people can selectively encode relevant features but not irrelevant features of the same visual object. Some experiments show that relevant and irrelevant features of the same object have similar behavioral effects on memory performance ( Marshall & Bays, 2013 ) and attentional capture ( Gao et al., 2016 ; see the section on effects of WM on perceptual attention for an explanation of this effect). However, one fMRI study found that the relevant but not the irrelevant feature of a visual object could be reconstructed from the pattern of BOLD activity during the retention interval ( Yu & Shim, 2017 ). Logie, Brockmole, and Jaswal ( 2011 ) have tested the effects of changes in irrelevant features on change-detection accuracy and found that such changes impair performance for retention intervals up to about 2 s but not thereafter. They propose that irrelevant features are initially encoded and subsequently removed from WM. This could explain why irrelevant features are not detectable in the sluggish BOLD signal that aggregates information over several seconds.
Filtering could be accomplished by perceptual selection – not attending to the irrelevant stimuli – but it could also be a separate selection step, such that a stimulus, even though selected for perceptual attention, is not encoded into WM. The latter possibility would imply that perceptual attention might be necessary, but is not sufficient for encoding them into WM. Evidence for this possibility comes from several sources. A series of experiments by H. Chen and Wyble ( 2015a , 2015b ) used stimuli as attentional cues for a perceptual decision task, and after several trials inserted a surprise memory test for a feature of the cue. Although they have arguably attended to the cue because it was relevant for the decision task, people had poor memory for its features only a few seconds after its disappearance, suggesting that the stimulus, or at least the feature probed in the memory test, was not encoded into WM. When people expected the memory test, their performance was much better. In a related experiment H. Chen, Swan, and Wyble ( 2016 ) had participants visually track several moving target objects among distractors. To avoid confusing the targets with distractors participants had to continuously attend to them while they moved. Yet, in a surprise memory test they had little memory for the target’s colors.
A second source of evidence suggesting that attention is not sufficient to encode stimuli into WM comes from some of my experiments ( Oberauer, 2018 ): Participants saw six words presented one by one in different screen locations; each word was followed by a cue to remember or forget it. The cue appeared only after word offset so that people had to attend to each word in case they would have to remember it. I also varied the time interval between each forget cue and the onset of the next word to manipulate how much time people had to remove a to-be-forgotten word from WM. The to-be-forgotten words had no effect on memory performance regardless of the cue-word interval, implying that they did not contribute at all to the load on WM.
These findings could mean that information, although attended, is not encoded into WM. Alternatively, the visual stimuli of Chen and Wyble, or the to-be-forgotten words in my experiments, could be encoded into WM but then removed very quickly so that their accessibility, and their effect on WM load, was not measurable even a few seconds later (see the section below on Removal). Perhaps neurophysiological markers of WM load with high temporal resolution, such as the CDA, could be leveraged to distinguish between these possibilities.
One limitation for efficient filtering (or removal) arises when people have to process the distracting material. When participants in my experiments ( Oberauer, 2018 ) had to make a judgment on each word while it was on the screen, they could not entirely prevent encoding to-be-forgotten words into WM, though they were still able to diminish their effect on WM load relative to to-be-remembered words. Marshall and Bays ( 2013 ) found that comparing two stimuli during the retention interval of a visual WM task impaired WM performance as much as adding two more stimuli to the memory set, suggesting that encoding of these stimuli into WM could not be prevented at all.
Selective Retrieval from Long-Term Memory. Much of the information we process in WM comes from long-term memory. For the WM system to work effectively, it has to retrieve information from long-term memory selectively, so that only information useful for the current task enters WM ( Oberauer, 2009 ). A demonstration of the effectiveness of this gating mechanism comes from experiments investigating the effect of previously acquired long-term memories on WM performance ( Oberauer, Awh, & Sutterer, 2017 ). We had participants learn 120 associations between everyday objects and randomly selected colors. In a subsequent WM test they had to maintain three object-color conjunctions on each trial, and reproduce each object’s color by selecting it on a color wheel. Some of the objects in the WM test were objects for which they had learned an associated color before. These objects could reoccur in the WM test with their learned color – in which case retrieving the associated color should facilitate WM performance – whereas others reoccurred with a new random color – in which case retrieving the color from long-term memory should interfere with WM performance. We found evidence for proactive facilitation, but against proactive interference, implying that information from long-term memory is used if and only if the information in WM was so poor that drawing on long-term memory could only make things better.
Removal of Information from WM. The selection of which information to hold in WM is also controlled after encoding: Information no longer relevant must be rapidly removed so that it does not clutter WM ( Hasher et al., 1999 ). There is a body of evidence showing that people can selectively remove no-longer relevant information from WM (for a review see Lewis-Peacock, Kessler, & Oberauer, 2018 ).
Removing an entire memory set when replacing it with a new one is a seamless and rapid process, though – as filtering – it is not perfect: Traces of the old memory set remain in WM, creating some mild proactive interference when items in the two sets are similar to each other ( Ralph et al., 2011 ; Tehan & Humphreys, 1998 ), and a congruency benefit when the two sets partially overlap, sharing the same items in the same contexts ( Oberauer, Souza, Druey, & Gade, 2013 ). Removal of a single item from the current memory set has been isolated experimentally as a process involved in WM updating ( Ecker, Oberauer, & Lewandowsky, 2014 ). By contrast, removal is much less efficient when it comes to removing more than one item from a memory set but less than all of them: People find it difficult to remove a random subset of several items from a memory set. For instance, when informed, after encoding a list of six words, that the words in positions 2, 3, and 5 could be forgotten, there was no evidence that they did so – successful removal of a subset of three words was found only when they were already clearly marked as a separate subset at encoding ( Oberauer, 2018 ). In sum, the efficiency of removal is limited by the ability to discriminate between to-be-maintained and to-be-removed contents of WM.
To conclude, the WM system is equipped with very efficient – though not perfect – mechanisms for controlling its contents through filtering perceptual input, selectively retrieving information from LTM, and removing no-longer relevant materials. Through these selection processes the cognitive system manages to usually have only the most relevant information for the current goal in WM.
How is Information selected within WM?
Selecting information to be held in WM is a form of selection, but it not necessarily selection of one piece of information at the exclusion of all others: We often hold multiple separate items in WM simultaneously. Sometimes we have to select a single item from the set currently held in WM as the input for a process, or as the object of mental manipulation. Our ability to select individual items from the set currently held in WM points to a selection mechanism that I refer to as the focus of attention in WM ( Oberauer, 2002 ; Oberauer & Hein, 2012 ). Evidence for the operation of such a narrow selection mechanism within WM comes from three observations: (1) In short-term recognition tests the last-presented item in a list is accessed at a faster rate than preceding items, and this has been interpreted as showing that the last-encoded item remains in the focus of attention (for a review McElree, 2006 ). (2) When an item in WM is needed as input for a cognitive operation (e.g., adding or subtracting a number from a particular digit in WM), or when one item needs to be selected as the object of an updating operation (e.g., replacing an item in WM by a new stimulus), then operating on the same WM item again in the next step takes less time than selecting another item from the memory set for the next operation. This item-switch cost (or item-repetition benefit) has been explained by assuming that the object of a cognitive operation remains in the focus of attention after the operation has been completed, and therefore does not need to be selected again when the same object is required for the next operation ( Garavan, 1998 ; Oberauer, 2003 ). (3) After encoding a set of stimuli into WM, a retro-cue presented one to several seconds into the retention interval can guide attention to one item and thereby improve memory performance when that item is tested – often at the expense of performance when another item is tested ( Griffin & Nobre, 2003 ; Landman, Spekreijse, & Lamme, 2003 ; for a review see Souza & Oberauer, 2016 ).
Whereas most of these empirical demonstrations come from situations in which a single item in WM needs to be selected, it has been argued that the focus of attention can hold more than one item ( Gilchrist & Cowan, 2011 ). From the perspective of attention as selection, this should be feasible to the extent that selecting multiple items simultaneously does not undercut the purpose of selection. For instance, if the task is to update one out of several digits in WM through an arithmetic operation, selecting more than that one digit into the focus of attention would only lead to confusion – but if the task is to add two digits in WM together, selecting both of them into the focus of attention at the same time is arguably useful because then they could be used simultaneously as retrieval cues for the relevant arithmetic fact ( Oberauer, 2013 ). Another situation in which it is functional to select two items into the focus simultaneously is when two tasks must be carried out simultaneously, one on each item, and the two items are sufficiently different to not risk cross-talk between the two tasks ( Göthe, Oberauer, & Kliegl, 2016 ; Oberauer & Bialkova, 2011 ).
Using the retro-cue paradigm, neuroscience research has revealed a distinction between attended and unattended information in WM 4 : Whereas the attended information can be decoded from neural signals such as the pattern of BOLD activity over voxels, or the pattern of EEG activity over electrodes, the unattended information cannot – it remains neurally silent, but can be brought back into a neurally active state later by a retro-cue drawing attention to it ( LaRocque, Lewis-Peacock, Drysdale, Oberauer, & Postle, 2013 ; Lewis-Peacock, Drysdale, Oberauer, & Postle, 2011 ; Sprague, Ester, & Serences, 2016 ) or by an uninformative strong input to the cortex ( Rose et al., 2016 ; Wolff, Jochim, Akyürek, & Stokes, 2017 ). One recent study, however, paints a more differentiated picture: Decoding of orientations maintained in VWM from fMRI signals in visual cortex was again good for attended and absent for unattended items, but decoding from signals in parietal cortex (IPS and frontal eye fields) was equally good for both attended and unattended items – though much weaker than decoding of attended items in visual cortex ( Christophel, Iamshchinina, Yan, Allefeld, & Haynes, 2018 ).
Behavioral evidence shows that retro-cues can be used to select not just individual items but also subsets of several items within WM ( Oberauer, 2001 , 2005 ), and selection of a subset can be followed by selection of an item within that subset ( Oberauer, 2002 ). Therefore, we can distinguish three levels of selection in WM: (1) Selecting information to be in WM, constituting the current memory set, (2) selecting a subset of the memory set, and (3) selecting a single item from that subset. I have referred to these three levels as (1) the activated part of long-term memory, (2) the region of direct access, and (3) the focus of attention, respectively (see Oberauer, 2009 , for a detailed discussion of the 3-level framework and evidence supporting it; and Oberauer et al., 2013 , for a computational implementation). It is currently not clear whether more than one WM representation is neurally active (i.e., decodable from neural activity during the retention interval) at the same time, so we do not know whether the state of being neurally active characterizes the second or the third level of selection. One possibility is that during WM maintenance multiple representations – those in the direct-access region – are active at the same time, such that their pattern of neural activity is superimposed. Another possibility is that only one item – the one in the focus of attention – is neurally active at any time. If the focus of attention circulates among the items in WM, it would still be possible to decode several items from neural activation patterns ( Emrich, Rigall, LaRocque, & Postle, 2013 ) because the temporal resolution of decoding from BOLD signals is lower than the speed at which the focus of attention shifts from one item to another (i.e., about 300 ms; Oberauer, 2003 ).
Univariate neural correlates of WM load, most notably the amplitude of the CDA ( Vogel & Machizawa, 2004 ) and the BOLD activation in the inter-parietal sulcus (IPS) ( Todd & Marois, 2004 , 2005 ; Xu & Chun, 2006 ), imply that at least some form of persistent neural activity increases with the number of items maintained in WM. These neural measures, however, do not carry information about the content of WM, and therefore we do not know whether they reflect neurally active representations or some neural activity reflecting control processes that are involved in maintaining items selected. Another open question is whether these univariate measures of WM load reflect the first or the second level of selection – to find out we need studies that track these neural indicators of WM load while a retro-cue asks participants to select a subset of the current memory set: Does the neural marker track the set size of the subset or of the entire memory set? One study asking this question found that BOLD activation in IPS reflects the size of the entire memory set before the retro-cue but the size of the cued subset afterwards ( Lepsien, Thornton, & Nobre, 2011 ), suggesting that IPS activation reflects the second level of selection, the direct-access region. In that study, however, participants were not asked to still maintain the not-cued subset in memory, so we don’t know whether they maintained it (at the third selection level, the activated part of LTM) or just removed it from WM.
A somewhat speculative hypothesis on how to reconcile all these findings is that univariate markers of WM load track the amount of information selected at the second level (i.e., the direct-access region). This information is maintained in WM through temporary bindings between contents and contexts through which they are accessible, probably in parietal cortex. These bindings are neurally silent – either because they are implemented through rapid synaptic plasticity ( Mongillo, Barak, & Tsodyks, 2008 ) or because they are implemented in a pattern of neural activity that bears no similarity to the bound contents, such as a circular convolution of each content with its context ( Eliasmith, 2013 ; Plate, 2003 ), so that they cannot be identified through decoding of the WM contents. However, neural activity patterns corresponding to the contents of the direct-access region could be re-activated during the retention interval by feeding non-specific activation into the contexts that act as retrieval cues for these contents, so that they could (faintly) be decoded from parietal cortical areas ( Bettencourt & Xu, 2016 ; Christophel et al., 2018 ). This non-specific activation could be spontaneous noise in the neural network ( Oberauer & Lin, 2017 ), or an attentional mechanism that selectively activates all contexts to which the contents of the direct-access region are bound. The content (or contents) selected for the third level of selection, the focus of attention, is represented in a neurally active fashion, probably in the prefrontal cortex ( Bichot, Heard, DeGennaro, & Desimone, 2015 ; Mendoza-Halliday & Martinez-Trujillo, 2017 ), and this representation re-activates the corresponding sensory representation in those sensory cortical areas involved in its initial processing, so that the information in the focus of attention can be decoded from neural activity in those areas.
A prediction from this hypothesis is that when two to-be-remembered stimuli are presented sequentially, univariate markers such as the CDA should add up to reflect the combined load of both stimuli, whereas the decodability of the first stimulus should be substantially impaired by the encoding of the second, because the focus of attention abandons the first to encode the second stimulus. Evidence for the first assumption comes from studies showing that the CDA reflects the combined load of two successively presented parts of a memory set ( Feldmann-Wüstefeld, Vogel, & Awh, 2018 ; Ikkai, McCollough, & Vogel, 2010 ); the second prediction remains to be tested.
What is the Relation between WM and Perceptual Attention?
An extreme position would be that WM and perceptual attention are the same: By virtue of attending to a perceived stimulus, it is selected into WM. Maintaining stimuli in WM that are no longer present in the environment differs from perceptual attention only in the absence of the physical stimulus. The cognitive state is still the same, with the only difference that the representation in WM is arguably weaker and less precise due to the lack of informative sensory input. This extreme position is attractive due to its parsimony, but it is almost certainly wrong. We have already seen that perceptual attention to stimuli during the retention interval of a visual WM task leads to less interference than adding the same stimuli to WM ( Fougnie & Marois, 2006 ). I have also discussed instances where stimuli were attended to and yet they leave hardly any trace in WM (H. Chen et al., 2016 ; H. Chen & Wyble, 2015a , 2015b ; Oberauer, 2018 ). Moreover, single-cell recordings from monkey LPFC neurons showed partial but not complete overlap between the neurons responding selectively to a feature while it is perceptually attended and those doing so while the feature is being held in WM ( Mendoza-Halliday & Martinez-Trujillo, 2017 ). If we accept that perceptual attention and WM are different entities, we can meaningfully ask how they causally affect each other.
How does perceptual attention affect WM? Some authors have argued that perceptual attention can be used to rehearse visual or spatial WM contents. The evidence for this idea is mixed. Some studies found a correlation between spontaneous eye movements during the retention interval – which presumably track visual attention – and recall success for sequences of spatial locations ( Tremblay, Saint-Aubin, & Jalberg, 2006 ), but no such correlation was found for change detection in visual arrays ( Williams, Pouget, Boucher, & Woodman, 2013 ). Directing people to attend to individual items in a visual array improves memory for those items relative to not-attended items in the array ( Souza, Rerko, & Oberauer, 2015 ; Souza, Vergauwe, & Oberauer, 2018 ). However, it is not clear whether this effect relies on perceptual attention. Engaging perceptual attention by a secondary task during the retention interval (i.e., detection of a slight brightness change in the fixation cross) impaired performance in a visual change-detection task ( Williams et al., 2013 ), but had at best a negligible effect on errors in a visual continuous-reproduction task, whereas engaging central attention impaired continuous reproduction more severely ( Souza & Oberauer, 2017 ).
As discussed above in the section on Filtering, perceptual attention is probably necessary but not sufficient for encoding of stimuli into WM. Yet, filtering is not perfect, so that attended information is sometimes encoded into WM to some extent even when this is not desired. To the extent that this happens, we can expect that distractors presented during the retention interval of a WM task interfere with the to-be-remembered information, thereby impairing memory performance.
Evidence for such interference comes from studies of spatial WM. Van der Stigchel, Merten, Meeter, and Theeuwes ( 2007 ) found that recall of locations is biased towards the location of a suddenly appearing irrelevant stimulus on the screen, suggesting that this stimulus was inadvertently encoded into WM. Lawrence, Myerson, and Abrams ( 2004 ) had participants identify and compare two symbols during the retention interval of a WM task, which either appeared at fixation or in the periphery (left or right of fixation). When the symbols appeared in the periphery, spatial (but not verbal) WM performance was impaired more than for centrally displayed symbols. This suggests that attending to additional locations entails encoding these locations into WM to some degree, thereby interfering with memory for other locations. The interfering effect was stronger when participants were instructed to move their eyes to the peripheral symbols than when they were instructed to maintain fixation, in line with other findings showing that processing distractors enforces stronger encoding into WM than merely attending to them ( Oberauer, 2018 ). Both studies unfortunately lack a control condition in which irrelevant stimuli are presented but not attended, so it is not clear how much perceptual attention contributes to their encoding into WM.
Does attending to a stimulus in the environment distract the focus of attention from information in WM? Two observations indicate that it might not: The beneficial effect of a retro-cue directing the focus of attention to one item in WM is not diminished by a subsequent task engaging perceptual attention ( Hollingworth & Maxcey-Richard, 2013 ; Rerko, Souza, & Oberauer, 2014 ). Likewise, the object-repetition benefit in a spatial WM updating task was not diminished by requiring people to focus visual attention on a stimulus in the periphery in between updating steps ( Hedge, Oberauer, & Leonards, 2015 ). However, the retro-cue effect probably arises in part from strengthening of the cued item’s binding to its context, and this effect lasts after the focus of attention has moved away from the cued item ( Rerko et al., 2014 ; Souza et al., 2015 ). The same could be true for the object-repetition benefit: The item to be updated is selected into the focus of attention, and this strengthens the item’s binding to its context as a side effect, leaving that item temporarily more accessible than other items even if the focus of attention moves away from it. Evidence suggesting that attending to perceptual stimuli does distract the focus of attention comes from studies using multivariate neural signals to read out the information in the pattern of neural activity. The decodability of a single item in WM is drastically diminished – at least temporarily – by the onset of an irrelevant stimulus, or just by the person attending to a location in anticipation of a stimulus, during the retention interval ( Bettencourt & Xu, 2016 ; van Moorselaar et al., 2017 ). However, in these studies the irrelevant stimulus hardly affected memory performance. Therefore, an alternative possibility is that the content of the focus of attention is represented in pre-frontal cortex ( Bichot et al., 2015 ), and the corresponding sensory representations are merely epiphenomenal, so that the elimination of the latter does not imply a distraction of the focus of attention in WM.
To conclude, surprisingly little can be said with confidence: Perceptual attention to stimuli often – but not always – leads to them being encoded into WM to some extent, so that they interfere with similar information. The use of perceptual attention for rehearsal has not been demonstrated convincingly. Whether the focus of attention can stay on an item in WM while perceptual attention engages with a different stimulus in the environment is still unclear.
How does information in WM affect perceptual attention? It appears plausible that holding some information in WM tends to draw perceptual attention to similar information in the environment, thereby facilitating its processing. Initial evidence for that assumption comes from experiments by Awh et al. ( 1998 ): Holding the spatial location of an object in WM facilitates processing of other stimuli appearing in the same location during the retention interval. A subsequent similar study taking additional measures to discourage eye movements, however, failed to replicate this finding ( Belopolsky & Theeuwes, 2009 ).
A more specific version of the same idea is the assumption that the item held in the focus of attention in WM – usually a single item – functions as a “search template”, guiding perceptual attention to matching stimuli ( Olivers, Peters, Houtkamp, & Roelfsema, 2011 ). This idea has received considerable empirical support from studies of the “attentional capture” effect in visual search: When people are asked to hold an item in WM – for instance a color, or just a color word – and carry out a visual search task during the retention interval, attention is drawn to stimuli in the search display matching the item in WM ( Soto, Hodsoll, Rotshtein, & Humphreys, 2008 ). When more than one item is held in WM and one of them is retro-cued, then only the retro-cued item causes attentional capture ( Mallett & Lewis-Peacock, 2018 ; van Moorselaar, Battistoni, Theeuwes, & Olivers, 2014 ; van Moorselaar, Theeuwes, & Olivers, 2014 ). This finding provides further evidence for the special functional status of representations in the focus of attention (i.e., the third level of selection).
How is WM related to the control of attention and action?
Some theorists argue for a close relation of WM specifically to controlled attention ( Kane et al., 2001 ; McVay & Kane, 2009 ; Unsworth et al., 2014 ). The evidence for this link comes primarily from correlations between measures of WM capacity and controlled attention (reviewed above in the section on resources for attention control). There are at least two interpretations of this correlation. One is that people with high ability to control their attention are good at keeping irrelevant contents out of WM ( Hasher & Zacks, 1988 ), either by filtering them out at encoding ( Vogel et al., 2005 ) or by removing them once they are no longer relevant ( Oberauer et al., 2012 ), and therefore they make better use of their WM capacity. This account has difficulties explaining why measures of controlled attention were found to correlate substantially also with measures of (visual) WM in which no irrelevant stimuli were presented, and no contents need to be removed from WM ( Unsworth et al., 2014 ).
A second explanation, which I believe to be more promising, implies the reverse direction of causality. It starts from the assumption that the main function of WM is to hold representations that control what we think and do, including what we direct our attention to ( Oberauer, 2009 ). For instance, in visual search perceptual attention can be controlled by holding a template of the search target in the focus of attention in WM ( Olivers et al., 2011 ). Selection of responses to stimuli in accordance with the currently relevant task goal is accomplished by holding a task set – a representation of the relevant stimulus categories, the response options, and the mapping between them – in WM ( Monsell, 2003 ; Oberauer et al., 2013 ). In both cases, control could also rely on representations in long-term memory. For the case of visual search, Woodman, Carlisle, and Reinhart ( 2013 ) present strong evidence that search targets that repeat across successive trials are held in WM only for the first few trials, after which search is controlled by target representations in long-term memory. The finding that search becomes more efficient with practice when the same set of stimuli is consistently used as targets or distractors further underscores the role of long-term memory in controlling perceptual attention in search tasks ( Shiffrin & Schneider, 1977 ). For the case of response selection, practicing a task with consistent stimulus-response mappings leads to long-term learning of these mappings, greatly improving task performance. Representations in WM are necessary for control when we want to do something new – searching for a new target, or carrying out a new task that we just learned from instruction. WM representations are particularly important when the new action is inconsistent with one that we have learned – for instance, searching for a target that used to consistently figure as distractor, or switching from one task to another that maps the same stimuli to new responses. In these cases, WM provides a medium for building and maintaining new representations that control our cognitive processes and actions, if necessary countermanding our long-term knowledge. On these assumptions, the correlation between WM capacity and performance in controlled-attention tasks arises because people with better WM capacity have better (i.e., more robust, more precise) representations in WM of the (cognitive or overt) action they intend to carry out, such as search templates and task sets.
To conclude, I argue that WM plays a crucial role in controlling attention and action by holding the representations that guide attention and action. The control process consists of selecting these representations into WM – once they are established in WM, they have their influence on attention and action automatically: Perceptual attention is “captured” by stimuli matching the content of the focus of attention even when this is only detrimental to performance in the current task ( Foerster & Schneider, 2018 ; Gao et al., 2016 ); newly instructed tasks, once implemented as task sets in WM, function like a “prepared reflex”, influencing response selection even when they are currently not relevant ( Meiran, Liefooghe, & De Houwer, 2017 ).
Conclusions
Attention is closely related to WM. Unpacking this relationship reveals many different ways in which the WM-attention link can be spelled out. A first divide is between theoretical ideas about attention as a resource on the one hand, and about attention as a mechanism for selecting and prioritizing information on the other. The first approach entails the theoretical commitment that a limited attentional resource is at least in part responsible for the capacity limit of WM. This assumption has considerable empirical support but also significant weaknesses (for a review see Oberauer et al., 2016 ), so that researchers should not endorse it as a default. The second approach does not imply a commitment to any assumptions about WM or attention, and therefore offers a more neutral starting point for asking how the two are related. From the theoretical considerations and the evidence reviewed here I conclude that the following assertions about specific relations between attention and WM are justified:
By virtue of holding a selected subset of all available representations in memory, WM is by definition a form of attention.
The selection of information to be held in WM is a form of controlled attention: The selection of stimuli to be encoded into WM is controlled by a filtering mechanism set according to our intentions; the retrieval of long-term memory information into WM is gated to admit only information relevant for our current goals, and information no longer relevant for our current goal is removed from WM.
Attending to a perceived stimulus probably facilitates encoding of that stimulus into WM, but does not mandate it. Even attended information can be, to a large extent, filtered out.
Within the contents of WM the focus of attention can be directed to individual items, or subsets of items, selected for manipulating them, or as input for processes (e.g., mental arithmetic, visual search).
Control of attention and action relies on representations in WM that guide attention and action, such as search templates and task sets, especially when these are new and in conflict with knowledge in long-term memory. Once established in WM, these representations control attention and action independently of our intentions.
Unsurprisingly, there are also many things we don’t know. Table 2 presents a non-exhaustive list of open questions that I believe future research should address with high priority. I hope that this effort will lead to an increasingly more precise and nuanced picture of how WM is related to attention.
Open Questions.
Funding Statement
The work on this article was supported by a grant from the Swiss National Science Foundation (SNSF, grant number 100014_135002). Thanks to Peter Shepherdson and Claudia von Bastian for their comments on a previous version of this manuscript.
I will use the term object (of attention) in a broad sense, referring to every entity that we can pay attention to (e.g., physical objects, events, people, concepts and ideas, goals and actions, …).
Chun et al. ( 2011 ) refer to this distinction as “internal” vs. “external” attention. I find this terminology misleading: The memory of a tree is not more internal than the perception of a tree: Both are internal representations of external objects.
Another paradoxical implication of the fusion account is that, once the resource is completely absorbed for storage purposes, there is no resource left for control processes clearing irrelevant material from WM, and once an ongoing process monopolizes the entire attentional resource, there is no way of stopping it. A meta-control process is necessary to ensure that there is always enough resource left for control processes. If the meta-control process needs a share of the resource for itself, we are on the way to an infinite regress.
The term “unattended” is to be understood relative to the “attended” content of WM. At the same time, all contents of WM are prioritized over all other memory representations, and as such are attended, though on a broader level of selection.
Ethics and Consent
This article reports no original research, so no ethics approval is required.
Funding Information
Competing interests.
The author has no competing interests to declare.
- 1. Allen, R. J., Baddeley, A. D., & Hitch, G. J. (2006). Is the binding of visual features in working memory resource-demanding? Journal of Experimental Psychology: General, 135, 298–313. DOI: 10.1037/0096-3445.135.2.298 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Anderson, J. R., Reder, L. M., & Lebiere, C. (1996). Working memory: Activation limits on retrieval. Cognitive Psychology, 30, 221–256. DOI: 10.1006/cogp.1996.0007 [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Awh, E., Belopolsky, A. V., & Theeuwes, J. (2012). Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in Cognitive Sciences, 16, 437–443. DOI: 10.1016/j.tics.2012.06.010 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Awh, E., Jonides, J., & Reuter-Lorenz, P. A. (1998). Rehearsal in spatial working memory. Journal of Experimental Psychology: Human Perception and Performance, 24, 780–790. DOI: 10.1037/0096-1523.24.3.780 [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Baddeley, A. D. (1986). Working memory. Oxford: Clarendon Press. [ Google Scholar ]
- 6. Baddeley, A. D. (1993). Working memory or working attention? In: Baddeley A., & Weiskrantz L. (Eds.), Attention: Selection, awareness, and control, (pp. 152–170). Oxford: Clarendon. [ Google Scholar ]
- 7. Baddeley, A. D. (1996). Exploring the central executive. Quarterly Journal of Experimental Psychology, 49A, 5–28. DOI: 10.1080/713755608 [ DOI ] [ Google Scholar ]
- 8. Baddeley, A. D., Papagno, C., & Andrade, J. (1993). The sandwich effect: The role of attentional factors in serial recall. Journal of Experimental Psychology: Learning, Memory and Cognition, 19, 862–870. DOI: 10.1037/0278-7393.19.4.862 [ DOI ] [ Google Scholar ]
- 9. Barrouillet, P., Bernardin, S., & Camos, V. (2004). Time constraints and resource sharing in adults’ working memory spans. Journal of Experimental Psychology: General, 133, 83–100. DOI: 10.1037/0096-3445.133.1.83 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Barrouillet, P., Bernardin, S., Portrat, S., Vergauwe, E., & Camos, V. (2007). Time and cognitive load in working memory. Journal of Experimental Psychology: Learning, Memory & Cognition, 33, 570–585. DOI: 10.1037/0278-7393.33.3.570 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Barrouillet, P., Portrat, S., & Camos, V. (2011). On the law relating processing to storage in working memory. Psychological Review, 118, 175–192. DOI: 10.1037/a0022324 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Belopolsky, A. V., & Theeuwes, J. (2009). No functional role of attention-based rehearsal in maintenance of spatial working memory representations. Acta Psychologica, 132, 124–135. DOI: 10.1016/j.actpsy.2009.01.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Bettencourt, K. C., & Xu, Y. (2016). Decoding the content of visual short-term memory under distraction in occipital and parietal areas. Nature Neuroscience, 19, 150–157. DOI: 10.1038/nn.4174 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Bichot, N. P., Heard, M. T., DeGennaro, E. M., & Desimone, R. (2015). A Source for Feature-Based Attention in the Prefrontal Cortex. Neuron, 88(4), 832–844. DOI: 10.1016/j.neuron.2015.10.001 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Bunting, M. F., Cowan, N., & Colflesh, G. J. H. (2008). The deployment of attention in short-term memory tasks: Trade-offs between immediate and delayed deployment. Memory & Cognition, 36, 799–812. DOI: 10.3758/MC.36.4.799 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Camos, V., Lagner, P., & Barrouillet, P. (2009). Two maintenance mechanisms of verbal information in working memory. Journal of Memory and Language, 61, 457–469. DOI: 10.1016/j.jml.2009.06.002 [ DOI ] [ Google Scholar ]
- 17. Case, R. (1972). Validation of a neo-Piagetian mental capacity construct. Journal of Experimental Child Psychology, 14, 287–302. DOI: 10.1016/0022-0965(72)90051-3 [ DOI ] [ Google Scholar ]
- 18. Case, R., Kurland, M., & Goldberg, J. (1982). Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology, 33, 386–404. DOI: 10.1016/0022-0965(82)90054-6 [ DOI ] [ Google Scholar ]
- 19. Chein, J. M., Moore, A. B., & Conway, A. R. A. (2011). Domain-general mechanisms of complex working memory span. NeuroImage, 54, 550–559. DOI: 10.1016/j.neuroimage.2010.07.067 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Chen, H., Swan, G., & Wyble, B. (2016). Prolonged focal attention without binding: Trackng a ball for half a minute without remembering its color. Cognition, 147, 144–148. DOI: 10.1016/j.cognition.2015.11.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Chen, H., & Wyble, B. (2015a). Amnesia for object attributes: Failure to report attended information that had just reached conscious awareness. Psychological Science, 26, 203–210. DOI: 10.1177/0956797614560648 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Chen, H., & Wyble, B. (2015b). The location but not the attributes of visual cues are automatically encoded into working memory. Vision Research, 107, 76–85. DOI: 10.1016/j.visres.2014.11.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Chen, Z., & Cowan, N. (2009). How verbal memory loads consume attention. Memory & Cognition, 37, 829–836. DOI: 10.3758/MC.37.6.829 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Christophel, T. B., Iamshchinina, P., Yan, C., Allefeld, C., & Haynes, J.-D. (2018). Cortical specialization for attended versus unattended working memory. Nature Neuroscience, 21, 494–496. DOI: 10.1038/s41593-018-0094-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Chuderski, A. (2014). The relational integration task explains fluid reasoning above and beyond other working memory tasks. Memory & Cognition, 42, 448–463. DOI: 10.3758/s13421-013-0366-x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Chun, M. M. (2011). Visual working memory as visual attention sustained internally over time. Neuropsychologia, 49(6), 1407–1409. DOI: 10.1016/j.neuropsychologia.2011.01.029 [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Chun, M. M., Golomb, J. D., & Turk-Browne, N. B. (2011). A taxonomy of external and internal attention. Annual Review of Psychology, 62, 73–101. DOI: 10.1146/annurev.psych.093008.100427 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Cowan, N. (1995). Attention and memory: An integrated framework. New York: Oxford University Press. [ Google Scholar ]
- 29. Cowan, N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24, 87–185. DOI: 10.1017/S0140525X01003922 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Cowan, N. (2005). Working memory capacity. New York: Psychology Press. [ Google Scholar ]
- 31. Cowan, N. (2017). The many faces of working memory and short-term storage. Psychonomic Bulletin & Review, 24, 1158–1170. DOI: 10.3758/s13423-016-1191-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Cowan, N., Elliott, E. M., Saults, J. S., Morey, C. C., Mattox, S., Hismjatullina, A., & Conway, A. R. A. (2005). On the capacity of attention: its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology, 51, 42–100. DOI: 10.1016/j.cogpsych.2004.12.001 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Cowan, N., Fristoe, N., Elliott, E. M., Brunner, R. P., & Saults, J. S. (2006). Scope of attention, control of attention, and intelligence in children and adults. Memory & Cognition, 34, 1754–1768. DOI: 10.3758/BF03195936 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Daneman, M., & Carpenter, P. A. (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19, 450–466. DOI: 10.1016/S0022-5371(80)90312-6 [ DOI ] [ Google Scholar ]
- 35. Desimone, R., & Duncan, J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. DOI: 10.1146/annurev.ne.18.030195.001205 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Ecker, U. K. H., Lewandowsky, S., & Oberauer, K. (2014). Removal of information from working memory: A specific updating process. Journal of Memory and Language, 74, 77–90. DOI: 10.1016/j.jml.2013.09.003 [ DOI ] [ Google Scholar ]
- 37. Ecker, U. K. H., Oberauer, K., & Lewandowsky, S. (2014). Working memory updating involves item-specific removal. Journal of Memory & Language, 74, 1–15. DOI: 10.1016/j.jml.2014.03.006 [ DOI ] [ Google Scholar ]
- 38. Eliasmith, C. (2013). How to build a brain: A neural architecture for biological cognition. New York, NY: Oxford University Press; DOI: 10.1093/acprof:oso/9780199794546.001.0001 [ DOI ] [ Google Scholar ]
- 39. Emrich, S. M., Rigall, A. C., LaRocque, J. J., & Postle, B. R. (2013). Distributed patterns of activity in sensory cortex reflect the precision of multiple items maintained in visual short-term memory. Journal of Neuroscience, 33, 6516–6523. DOI: 10.1523/JNEUROSCI.5732-12.2013 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Ester, E. F., Fukuda, K., May, L. M., Vogel, E. K., & Awh, E. (2014). Evidence for a fixed capacity limit in attending multiple locations. Cognitive, Affective, & Behavioral Neuroscience, 14, 62–77. DOI: 10.3758/s13415-013-0222-2 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Feldmann-Wüstefeld, T., Vogel, E. K., & Awh, E. (2018). Contralateral delay activity indexes working memory storage, not the current focus of spatial attention. Journal of Cognitive Neuroscience, 30(8), 1185–1196. DOI: 10.1162/jocn_a_01271 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Foerster, R. M., & Schneider, W. X. (2018). Involuntary top-down control by search-irrelevant features: Visual working memory biases attention in an object-based manner. Cognition, 172, 37–45. DOI: 10.1016/j.cognition.2017.12.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Fougnie, D., & Marois, R. (2006). Distinct capacity limits for attention and working memory. Psychological Science, 17, 526–534. DOI: 10.1111/j.1467-9280.2006.01739.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Gao, Z., Yu, S., Zhu, C., Shui, R., Weng, X., Li, P., & Shen, M. (2016). Object-based encoding in visual working memory: Evidence from memory-driven attentional capture. Scientific Reports, 6 DOI: 10.1038/srep22822 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Garavan, H. (1998). Serial attention within working memory. Memory & Cognition, 26, 263–276. DOI: 10.3758/BF03201138 [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Gazzaley, A., & Nobre, A. C. (2012). Top-down modulation: bridging selective attention and working memory. Trends in Cognitive Sciences, 16, 129–135. DOI: 10.1016/j.tics.2011.11.014 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Gilchrist, A. L., & Cowan, N. (2011). Can the focus of attention accommodate multiple, separate items? Journal of Experimental Psychology: Learning, Memory, and Cognition, 37, 1484–1502. DOI: 10.1037/a0024352 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Göthe, K., Oberauer, K., & Kliegl, R. (2016). Eliminating dual-task costs by minimizing crosstalk between tasks: The role of modality and feature pairings. Cognition, 150, 92–108. DOI: 10.1016/j.cognition.2016.02.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Griffin, I. C., & Nobre, A. C. (2003). Orienting attention to locations in internal representations. Journal of Cognitive Neuroscience, 15, 1176–1194. DOI: 10.1162/089892903322598139 [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Hasher, L., & Zacks, R. T. (1988). Working memory, comprehension, and aging: A review and a new view In: Bower G. H. (Ed.), The Psychology of Learning and Motivation, 22, (pp. 193–225). New York: Academic Press; DOI: 10.1016/S0079-7421(08)60041-9 [ DOI ] [ Google Scholar ]
- 51. Hasher, L., Zacks, R. T., & May, C. P. (1999). Inhibitory control, circadian arousal, and age In: Gopher D., & Koriat A. (Eds.), Attention and Performance, (pp. 653–675). Cambridge, MA: MIT Press. [ Google Scholar ]
- 52. Hazeltine, E., & Witfall, T. (2011). Searching working memory for the source of dual-task costs. Psychological Research, 75, 466–475. DOI: 10.1007/s00426-011-0343-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Hedge, C., Oberauer, K., & Leonards, U. (2015). Selection in spatial working memory is independent of perceptual selective attention, but they interact in a shared spatial priority map. Attention, Perception & Psychophysics, 77, 26653–22668. DOI: 10.3758/s13414-015-0976-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Hollingworth, A., & Beck, V. M. (2016). Memory-based attention capture when multiple items are maintained in visual working memory. Journal of Experimental Psychology: Human Perception and Performance, 42, 911–917. DOI: 10.1037/xhp0000230 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Hollingworth, A., & Maxcey-Richard, A. M. (2013). Selective maintenance in visual working memory does not require sustained visual attention. Journal of Experimental Psychology: Human Perception and Performance, 39, 1047–1058. DOI: 10.1037/a0030238 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Ikkai, A., McCollough, A. W., & Vogel, E. K. (2010). Contralateral delay activity provides a neural measure of the number of representations in visual working memory. Journal of Neurophysiology, 103, 1963–1968. DOI: 10.1152/jn.00978.2009 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Jolicoeur, P., & Dell’Acqua, R. (1998). The demonstration of short-term consolidation. Cognitive Psychology, 36, 138–202. DOI: 10.1006/cogp.1998.0684 [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Jurado, M. B., & Rosselli, M. (2007). The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review, 17, 213–233. DOI: 10.1007/s11065-007-9040-z [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Just, M. A., & Carpenter, P. A. (1980). A theory of reading: From eye fixations to comprehension. Psychological Review, 87, 329–354. DOI: 10.1037/0033-295X.87.4.329 [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Just, M. A., & Carpenter, P. A. (1992). A capacity theory of comprehension: Individual differences in working memory. Psychological Review, 99, 122–149. DOI: 10.1037/0033-295X.99.1.122 [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Kane, M. J., Bleckley, M. K., Conway, A. R. A., & Engle, R. W. (2001). A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General, 130, 169–183. DOI: 10.1037/0096-3445.130.2.169 [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Kane, M. J., & Engle, R. W. (2003). Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General, 132(47–70). DOI: 10.1037/0096-3445.132.1.47 [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Kelley, T. A., & Lavie, N. (2011). Working Memory Load Modulates Distractor Competition in Primary Visual Cortex. Cerebral Cortex, 21(3), 659–665. DOI: 10.1093/cercor/bhq139 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Keye, D., Wilhelm, O., Oberauer, K., & van Ravenzwaaij, D. (2009). Individual differences in conflict-monitoring: Testing means and covariance hypothesis about the Simon and the Eriksen Flanker task. Psychological Research-Psychologische Forschung, 73(6), 762–776. DOI: 10.1007/s00426-008-0188-9 [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Kim, S.-Y., Kim, M.-S., & Chun, M. M. (2005). Concurrent working memory load can reduce distraction. Proceedings of the National Academy of Sciences, 102, 16524–16529. DOI: 10.1073/pnas.0505454102 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Kiyonaga, A., & Egner, T. (2014). Working memory as internal attention: Toward an integrative account of internal and external selection processes. Psychonomic Bulletin & Review. DOI: 10.3758/s13423-012-0359-y [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Klapp, S. T., Marshburn, E. A., & Lester, P. T. (1983). Short-term memory does not involve the “working memory” of information processing: The demise of a common assumption. Journal of Experimental Psychology: General, 112, 240–264. DOI: 10.1037/0096-3445.112.2.240 [ DOI ] [ Google Scholar ]
- 68. Konstantinou, N., Beal, E., King, J.-R., & Lavie, N. (2014). Working memory load and distraction: Dissociable effects of visual maintenance and cognitive control. Attention, Perception & Psychophysics, 76, 1985–1997. DOI: 10.3758/s13414-014-0742-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Konstantinou, N., & Lavie, N. (2013). Dissociable roles of different types of working memory load in visual detection. Journal of Experimental Psychology: Human Perception and Performance, 39, 919–924. DOI: 10.1037/a0033037 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Landman, R., Spekreijse, H., & Lamme, V. A. F. (2003). Large capacity storage of integrated objects before change blindness. Vision Research, 43(149–164). DOI: 10.1016/S0042-6989(02)00402-9 [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. LaRocque, J. J., Lewis-Peacock, J. A., Drysdale, A. T., Oberauer, K., & Postle, B. R. (2013). Decoding attended information in short-term memory: An EEG study. Journal of Cognitive Neuroscience, 25(1), 127–142. DOI: 10.1162/jocn_a_00305 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Lavie, N. (2005). Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences, 9, 75–82. DOI: 10.1016/j.tics.2004.12.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Lavie, N., Hirst, A., de Fockert, J. W., & Viding, E. (2004). Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General, 133, 339–354. DOI: 10.1037/0096-3445.133.3.339 [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Lawrence, B. M., Myerson, J., & Abrams, R. A. (2004). Interference with spatial working memory: An eye movement is more than a shift of attention. Psychonomic Bulletin & Review, 11, 488–494. DOI: 10.3758/BF03196600 [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Lawrence, B. M., Myerson, J., Oonk, H. M., & Abrams, R. A. (2001). The effects of eye and limb movements on working memory. Memory, 9, 433–444. DOI: 10.1080/09658210143000047 [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Lepsien, J., Thornton, I., & Nobre, A. C. (2011). Modulation of working-memory maintenance by directed attention. Neuropsychologia, 49, 1569–1577. DOI: 10.1016/j.neuropsychologia.2011.03.011 [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Lewis-Peacock, J. A., Drysdale, A. T., Oberauer, K., & Postle, B. R. (2011). Neural evidence for a distinction between short-term memory and the focus of attention. Journal of Cognitive Neuroscience, 24, 61–79. DOI: 10.1162/jocn_a_00140 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Lewis-Peacock, J. A., Kessler, Y., & Oberauer, K. (2018). The removal of information from working memory. Annals of the New York Academy of Science, 1424, 33–44. DOI: 10.1111/nyas.13714 [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Liefooghe, B., Barrouillet, P., Vandierendonck, A., & Camos, V. (2008). Working memory costs of task switching. Journal of Experimental Psychology: Learning, Memory, and Cognition, 34, 478–494. DOI: 10.1037/0278-7393.34.3.478 [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Logie, R. H., Brockmole, J. B., & Jaswal, S. (2011). Feature binding in visual short-term memory is unaffected by task-irrelevant changes of location, shape, and color. Memory & Cognition, 39, 24–36. DOI: 10.3758/s13421-010-0001-z [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Luria, R., Balaban, H., Awh, E., & Vogel, E. K. (2016). The contralateral delay activity as a neural measure of visual working memory. Neuroscience and Biobehavioral Reviews, 62, 100–108. DOI: 10.1016/j.neubiorev.2016.01.003 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Ma, W. J., Husain, M., & Bays, P. M. (2014). Changing concepts of working memory. Nature Neuroscience Reviews, 17, 347–356. DOI: 10.1038/nn.3655 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Mall, J. T., Morey, C. C., Wolff, M. J., & Lehnert, F. (2014). Visual selective attention is equally functional for individuals with low and high working memory capacity: Evidence from accuracy and eye movements. Attention, Perception & Psychophysics. DOI: 10.3758/s13414-013-0610-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Mallett, R., & Lewis-Peacock, J. A. (2018). Behavioral decoding of working memory items inside and outside the focus of attention. Annals of the New York Academy of Sciences, 1424(1), 256–267. DOI: 10.1111/nyas.13647 [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Marshall, L., & Bays, P. M. (2013). Obligatory encoding of task-irrelevant features depletes working memory resources. Journal of Vision, 13, 1–13. DOI: 10.1167/13.2.21 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. McElree, B. (2006). Accessing recent events In: Ross B. H. (Ed.), The Psychology of Learning and Motivation, 46, (pp. 155–200). San Diego: Academic Press; DOI: 10.1016/S0079-7421(06)46005-9 [ DOI ] [ Google Scholar ]
- 87. McVay, J. C., & Kane, M. J. (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35, 196–204. DOI: 10.1037/a0014104 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. McVay, J. C., & Kane, M. J. (2012). Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General, 141, 302–320. DOI: 10.1037/a0025250 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Meiran, N., Liefooghe, B., & De Houwer, J. (2017). Powerful instructions: Automaticity without practice. Current Directions in Psychological Science, 26, 509–514. DOI: 10.1177/0963721417711638 [ DOI ] [ Google Scholar ]
- 90. Mendoza-Halliday, D., & Martinez-Trujillo, J. C. (2017). Neuronal population coding of perceived and memorized visual features in the lateral prefrontal cortex. nature Communications, 8, 15471 DOI: 10.1038/ncomms15471 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Mongillo, G., Barak, O., & Tsodyks, M. (2008). Synaptic theory of working memory. Science, 319, 1543–1546. DOI: 10.1126/science.1150769 [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140. DOI: 10.1016/S1364-6613(03)00028-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Morey, C. C., & Bieler, M. (2012). Visual short-term memory always requires general attention. Psychonomic Bulletin & Review, 20, 163–170. DOI: 10.3758/s13423-012-0313-z [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Navon, D., & Gopher, D. (1979). On the economy of the human-processing system. Psychological Review, 86, 214–255. DOI: 10.1037/0033-295X.86.3.214 [ DOI ] [ Google Scholar ]
- 95. Navon, D., & Miller, J. (2002). Queuing or sharing? A critical evaluation of the single-bottleneck notion. Cognitive Psychology, 44, 193–251. DOI: 10.1006/cogp.2001.0767 [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Nieuwenstein, M., & Wyble, B. (2014). Beyond a mask and against the bottleneck: Retroactive dual-task interference during working memory consolidation of a masked visual target. Journal of Experimental Psychology: General, 143, 1409–1427. DOI: 10.1037/a0035257 [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Oberauer, K. (2001). Removing irrelevant information from working memory: A cognitive aging study with the modified Sternberg task. Journal of Experimental Psychology-Learning Memory and Cognition, 27(4), 948–957. DOI: 10.1037/0278-7393.27.4.948 [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Oberauer, K. (2002). Access to information in working memory: Exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition, 28(3), 411–421. DOI: 10.1037/0278-7393.28.3.411 [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Oberauer, K. (2003). Selective attention to elements in working memory. Experimental Psychology, 50(4), 257–269. DOI: 10.1026//1618-3169.50.4.257 [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Oberauer, K. (2005). Control of the contents of working memory – A comparison of two paradigms and two age groups. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31(4), 714–728. DOI: 10.1037/0278-7393.31.4.714 [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Oberauer, K. (2009). Design for a working memory. Psychology of Learning and Motivation: Advances in Research and Theory, 51, 45–100. DOI: 10.1016/S0079-7421(09)51002-X [ DOI ] [ Google Scholar ]
- 102. Oberauer, K. (2013). The focus of attention in working memory – from metaphors to mechanisms. frontiers in human neuroscience, 7 DOI: 10.3389/fnhum.2013.00673 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Oberauer, K. (2018). Removal of irrelevant information from working memory: Sometimes fast, sometimes slow, and sometimes not at all. Annals of the New York Academy of Science, 1424, 239–255. DOI: 10.1111/nyas.13603 [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Oberauer, K., Awh, E., & Sutterer, D. W. (2017). The role of long-term memory in a test of visual working memory: Proactive facilitation but no proactive interference. Journal of Experimental Psychology: Learning, Memory and Cognition, 43, 1–22. DOI: 10.1037/xlm0000302 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Oberauer, K., & Bialkova, S. (2011). Serial and parallel processes in working memory after practice. Journal of Experimental Psychology: Human Perception and Performance, 37(2), 606–614. DOI: 10.1037/a0020986 [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Oberauer, K., Demmrich, A., Mayr, U., & Kliegl, R. (2001). Dissociating retention and access in working memory: An age-comparative study of mental arithmetic. Memory & Cognition, 29(1), 18–33. DOI: 10.3758/BF03195737 [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Oberauer, K., Farrell, S., Jarrold, C., & Lewandowsky, S. (2016). What limits working memory capacity? Psychological Bulletin, 142, 758–799. DOI: 10.1037/bul0000046 [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Oberauer, K., & Hein, L. (2012). Attention to information in working memory. Current Directions in Psychological Science, 21, 164–169. DOI: 10.1177/0963721412444727 [ DOI ] [ Google Scholar ]
- 109. Oberauer, K., & Lewandowsky, S. (2013). Evidence against decay in verbal working memory. Journal of Experimental Psychology: General, 142, 380–411. DOI: 10.1037/a0029588 [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Oberauer, K., & Lewandowsky, S. (2014). Further evidence against decay in working memory. Journal of Memory and Language, 73, 15–30. DOI: 10.1016/j.jml.2014.02.003 [ DOI ] [ Google Scholar ]
- 111. Oberauer, K., Lewandowsky, S., Farrell, S., Jarrold, C., & Greaves, M. (2012). Modeling working memory: An interference model of complex span. Psychonomic Bulletin & Review, 19, 779–819. DOI: 10.3758/s13423-012-0272-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Oberauer, K., & Lin, H.-Y. (2017). An interference model of visual working memory. Psychological Review, 124, 21–59. DOI: 10.1037/rev0000044 [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Oberauer, K., Souza, A. S., Druey, M., & Gade, M. (2013). Analogous mechanisms of selection and updating in declarative and procedural working memory: Experiments and a computational model. Cognitive Psychology, 66, 157–211. DOI: 10.1016/j.cogpsych.2012.11.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Olivers, C. N. L. (2008). Interactions between visual working memory and visual attention. Frontiers in Bioscience, 13, 1182–1191. DOI: 10.2741/2754 [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Olivers, C. N. L., Peters, J., Houtkamp, R., & Roelfsema, P. R. (2011). Different states in visual working memory: When it guides attention and when it does not. Trends in Cognitive Sciences, 15, 327–334. DOI: 10.1016/j.tics.2011.05.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Park, S., Kim, M.-S., & Chun, M. M. (2007). Concurrent working memory load can facilitate selective attention: Evidence for specialized load. Journal of Experimental Psychology: Human Perception and Performance, 33(1062–1075). DOI: 10.1037/0096-1523.33.5.1062 [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Pashler, H. (1994). Dual-task interference in simple tasks: Data and theory. Psychological Bulletin, 116, 220–244. DOI: 10.1037/0033-2909.116.2.220 [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Plate, T. A. (2003). Convolution-based memory models In: Nadel L. (Ed.), Encyclopedia of cognitive science, (pp. 824–828). London: Nature Publishing Group. [ Google Scholar ]
- 119. Ralph, A., Walters, J. N., Stevens, A., Fitzgerald, K. J., Tehan, G., Surprenant, A. M., Turcotte, J., et al. (2011). Immunity to proactive interference is not a property of the focus of attention in working memory. Memory & Cognition, 39, 217–230. DOI: 10.3758/s13421-010-0030-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Randall, J. G., Oswald, F. L., & Beier, M. E. (2014). Mind-wandering, cognition, and performance: A theory-driven meta-analysis of attention regulation. Psychological Bulletin, 140, 1411–1431. DOI: 10.1037/a0037428 [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Rerko, L., Souza, A. S., & Oberauer, K. (2014). Retro-cue benefits in working memory without sustained attention. Memory & Cognition, 42, 712–728. DOI: 10.3758/s13421-013-0392-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Ricker, T. J., & Hardman, K. O. (2017). The nature of short-term consolidation in visual working memory. Journal of Experimental Psychology: General. DOI: 10.1037/xge0000346 [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Rose, N. S., LaRocque, J. J., Riggall, A. C., Gosseries, O., Starrett, M. J., Meyering, E. E., & Postle, B. R. (2016). Reactivation of latent working memories with transcranial magnetic stimulation. Science, 354, 1136–1139. DOI: 10.1126/science.aah7011 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 124. Sander, M. C., Werkle-Bergner, M., & Lindenberger, U. (2011). Binding and strategic selection in working memory: A lifespan dissociation. Psychology and Aging, 26, 612–624. DOI: 10.1037/a0023055 [ DOI ] [ PubMed ] [ Google Scholar ]
- 125. Saults, J. S., & Cowan, N. (2007). A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. Journal of Experimental Psychology: General, 136, 663–684. DOI: 10.1037/0096-3445.136.4.663 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 126. Schneider, W., & Shiffrin, R. M. (1977). Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review, 84, 1–66. DOI: 10.1037/0033-295X.84.1.1 [ DOI ] [ Google Scholar ]
- 127. Shiffrin, R. M., & Schneider, W. (1977). Controlled and automatic human information processing: II. Perceptual learning, automatic attending, and a general theory. Psychological Review, 84, 127–190. DOI: 10.1037/0033-295X.84.2.127 [ DOI ] [ Google Scholar ]
- 128. Shipstead, Z., Lindsey, D. R. B., Marshall, R. L., & Engle, R. E. (2014). The mechanisms of working memory capacity: Primary memory, secondary memory, and attention control. Journal of Memory and Language, 72, 116–141. DOI: 10.1016/j.jml.2014.01.004 [ DOI ] [ Google Scholar ]
- 129. Singh, K. A., Gignac, G. E., Brydges, C. R., & Ecker, U. K. H. (2018). Working memory capacity mediates the relationship between removal and fluid intelligence. Journal of Memory and Language, 101, 18–36. DOI: 10.1016/j.jml.2018.03.002 [ DOI ] [ Google Scholar ]
- 130. Soto, D., Hodsoll, J., Rotshtein, P., & Humphreys, G. W. (2008). Automatic guidance of attention from working memory. Trends in Cognitive Sciences, 12, 342–348. DOI: 10.1016/j.tics.2008.05.007 [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Souza, A. S., & Oberauer, K. (2016). In search of the focus of attention in working memory: 13 years of the retro-cue effect. Attention, Perception & Psychophysics. DOI: 10.3758/s13414-016-1108-5 [ DOI ] [ PubMed ] [ Google Scholar ]
- 132. Souza, A. S., & Oberauer, K. (2017). The contributions of visual and central attention to visual working memory. Attention, Perception & Psychophysics, 79, 1897–1916. DOI: 10.3758/s13414-017-1357-y [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. Souza, A. S., Rerko, L., & Oberauer, K. (2015). Refreshing memory traces: Thinking of an item improves retrieval from visual working memory. Annals of the New York Academy of Sciences, 1339, 20–31. DOI: 10.1111/nyas.12603 [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Souza, A. S., Vergauwe, E., & Oberauer, K. (2018). Where to attend next: Guiding refreshing of visual, spatial, and verbal representations in working memory. Annals of the New York Academy of Science. DOI: 10.1111/nyas.13621 [ DOI ] [ PubMed ] [ Google Scholar ]
- 135. Sprague, T. C., Ester, E. F., & Serences, J. T. (2016). Restoring latent visual working memory representations in human cortex. Neuron, 91, 694–707. DOI: 10.1016/j.neuron.2016.07.006 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 136. Szmalec, A., Vandierendonck, A., & Kemps, E. (2005). Response selection involves executive control: Evidence from the selective interference paradigm. Memory & Cognition, 33, 531–541. DOI: 10.3758/BF03193069 [ DOI ] [ PubMed ] [ Google Scholar ]
- 137. Tehan, G., & Humphreys, M. S. (1998). Creating proactive interference in immediate recall: Building a DOG from a DART, a MOP, and a FIG. Memory & Cognition, 26, 477–489. DOI: 10.3758/BF03201157 [ DOI ] [ PubMed ] [ Google Scholar ]
- 138. Thalmann, M., Souza, A. S., & Oberauer, K. (2019). Revisiting the attentional demands of rehearsal in working-memory tasks. Journal of Memory and Language, 105, 1–18. DOI: 10.1016/j.jml.2018.10.005 [ DOI ] [ Google Scholar ]
- 139. Theeuwes, J. (2018). Visual selection: Usually fast and automatic; seldom slow and volitional. Journal of Cognition, 1, 1–15. DOI: 10.5334/joc.13 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 140. Todd, J. J., & Marois, R. (2004). Capacity limit of visual short-term memory in human posterior parietal cortex. Nature, 428, 751–753. DOI: 10.1038/nature02466 [ DOI ] [ PubMed ] [ Google Scholar ]
- 141. Todd, J. J., & Marois, R. (2005). Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cognitive, Affective, & Behavioral Neuroscience, 5, 144–155. DOI: 10.3758/CABN.5.2.144 [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Tombu, M., & Jolicoeur, P. (2003). A central capacity sharing model of dual-task performance. Journal of Experimental Psychology: Human Perception and Performance, 29, 3–18. DOI: 10.1037/0096-1523.29.1.3 [ DOI ] [ PubMed ] [ Google Scholar ]
- 143. Tremblay, S., Saint-Aubin, J., & Jalberg, A. (2006). Rehearsal in serial memory for visual-spatial information: Evidence from eye movements. Psychonomic Bulletin & Review, 13, 452–457. DOI: 10.3758/BF03193869 [ DOI ] [ PubMed ] [ Google Scholar ]
- 144. Tsubomi, H., Fukuda, K., Watanabe, K., & Vogel, E. K. (2013). Neural limits to representing objects still within view. Journal of Neuroscience, 33, 8257–8263. DOI: 10.1523/JNEUROSCI.5348-12.2013 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 145. Ueno, T., Allen, R. J., Baddeley, A. D., Hitch, G. J., & Saito, S. (2011). Disruption of visual feature binding in working meemory. Memory & Cognition, 39, 12–23. DOI: 10.3758/s13421-010-0013-8 [ DOI ] [ PubMed ] [ Google Scholar ]
- 146. Unsworth, N. (2015). Consistency of attentional control as an important cognitive trait: A latent variable analysis. Intelligence, 49, 110–128. DOI: 10.1016/j.intell.2015.01.005 [ DOI ] [ Google Scholar ]
- 147. Unsworth, N., Fukuda, K., Awh, E., & Vogel, E. K. (2014). Working memory and fluid intelligence: Capacity, attention control, and secondary memory retrieval. Cognitive Psychology, 71, 1–26. DOI: 10.1016/j.cogpsych.2014.01.003 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 148. Van der Stigchel, S., Merten, H., Meeter, M., & Theeuwes, J. (2007). The effects of a task-irrelevant visual event on spatial working memory. Psychonomic Bulletin & Review, 14, 1066–1071. DOI: 10.3758/BF03193092 [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. van Moorselaar, D., Battistoni, E., Theeuwes, J., & Olivers, C. N. L. (2014). Rapid influences of cued visual memories on attentional guidance. Annals of the New York Academy of Sciences. DOI: 10.1111/nyas.12574 [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. van Moorselaar, D., Foster, J. J., Sutterer, D. W., Theeuwes, J., Olivers, C. N. L., & Awh, E. (2017). Spatially selective alpha oscillations reveal moment-by-moment trade-offs between working memory and attention. Journal of Cognitive Neuroscience, 30, 256–266. DOI: 10.1162/jocn_a_01198 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 151. van Moorselaar, D., Theeuwes, J., & Olivers, C. N. L. (2014). In competition for the attentional template: Can multiple items within visual working memory guide attention? Journal of Experimental Psychology: Human Perception and Performance, 40, 1450–1464. DOI: 10.1037/a0036229 [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Vergauwe, E., Barrouillet, P., & Camos, V. (2010). Do mental processes share a domain-general resource? Psychological Science, 21, 384–390. DOI: 10.1177/0956797610361340 [ DOI ] [ PubMed ] [ Google Scholar ]
- 153. Vergauwe, E., Camos, V., & Barrouillet, P. (2014). The impact of storage on processing: How is information maintained in working memory? Journal of Experimental Psychology: Learning, Memory, and Cognition, 40, 1072–1095. DOI: 10.1037/a0035779 [ DOI ] [ PubMed ] [ Google Scholar ]
- 154. Vogel, E. K., & Machizawa, M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. DOI: 10.1038/nature02447 [ DOI ] [ PubMed ] [ Google Scholar ]
- 155. Vogel, E. K., McCollough, A. W., & Machizawa, M. G. (2005). Neural measures reveal individual differences in controlling access to working memory. Nature, 438(24), 500–503. DOI: 10.1038/nature04171 [ DOI ] [ PubMed ] [ Google Scholar ]
- 156. Watanabe, K., & Funahashi, S. (2014). Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nature Neuroscience, 17, 601–611. DOI: 10.1038/nn.3667 [ DOI ] [ PubMed ] [ Google Scholar ]
- 157. Wickens, C. D. (1980). The structure of attentional ressources In: Nickerson R. S. (Ed.), Attention & Performance, VIII, (pp. 239–257). Hillsdale, N.J.: Erlbaum. [ Google Scholar ]
- 158. Wilhelm, O., Hildebrandt, A., & Oberauer, K. (2013). What is working memory capacity, and how can we measure it? frontiers in Psychology, 4 DOI: 10.3389/fpsyg.2013.00433 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 159. Williams, M., Pouget, P., Boucher, L., & Woodman, G. F. (2013). Visual-spatial attention aids the maintenance of object representations in visual working memory. Memory & Cognition, 41, 698–715. DOI: 10.3758/s13421-013-0296-7 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 160. Wolff, M. J., Jochim, J., Akyürek, E. G., & Stokes, M. G. (2017). Dynamic hidden states underlying working-memory-guided behavior. Nature Neuroscience, 20, 864–871. DOI: 10.1038/nn.4546 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 161. Woodman, G. F., Carlisle, N. B., & Reinhart, R. M. G. (2013). Where do we store the memory representations that guide attention? Journal of Vision, 13, 1–17. DOI: 10.1167/13.3.1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 162. Woodman, G. F., & Chun, M. M. (2006). The role of working memory and long-term memory in visual search. Visual Cognition, 14, 808–830. DOI: 10.1080/13506280500197397 [ DOI ] [ Google Scholar ]
- 163. Xu, Y., & Chun, M. M. (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature, 440, 91–94. DOI: 10.1038/nature04262 [ DOI ] [ PubMed ] [ Google Scholar ]
- 164. Yeung, N., Botvinick, M., & Cohen, J. D. (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111, 931–959. DOI: 10.1037/0033-295X.111.4.931 [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Yu, Q., & Shim, W. M. (2017). Occipital, parietal, and frontal cortices selectively maintain task-relevant features of multi-feature objects in visual working memory. NeuroImage, 157, 97–107. DOI: 10.1016/j.neuroimage.2017.05.055 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.9 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
The Invisible Gorilla (Inattentional Blindness)

If you're here, you probably already know a bit about the Invisible Gorilla video and how it relates to attention. Did you know this experiment supports a fascinating concept called "inattentional blindness?"
What Is The Invisible Gorilla Experiment?

In 1999, Chris Chabris and Dan Simons conducted an experiment known as the “Invisible Gorilla Experiment.” They told participants they would watch a video of people passing around basketballs. In the middle of the video, a person in a gorilla suit walked through the circle momentarily.
The researchers asked participants if they would see the gorilla. Of course, they would, right? Not so fast. Before the researchers asked participants to watch the video, they asked them to count how many times people in the white shirts passed the basketball. In this initial experiment, 50% of the participants failed to see the gorilla!
The Invisible Gorilla and Inattentional Blindness
This case supports the existence of inattentional blindness (also known as perceptual blindness.) Chabris and Simons describe the research into this phenomenon in their 2010 book The Invisible Gorilla: How Our Intuitions Deceive Us . The book also describes the serious effect inattentional blindness can have on court cases, our perception of ourselves, and even life and death.

We don’t think that we fail to notice things. After all, if we fail to notice something...we go about our day without noticing that we didn’t notice something. The gorilla experiment shakes this idea up and often makes people uncomfortable.
What is Inattentional Blindness?
Inattentional blindness is a type of blindness that has nothing to do with your ability to see. It has to do with your ability to pay attention to unexpected stimuli. If your capacity is limited to one task or set of stimuli, you may fail to see the unexpected stimuli, even if it’s right in front of you.
Chabris and Simons were not the first psychologists to research this phenomenon. Arien Mack and Irvin Rock coined the term “inattentional blindness” in 1992 and published a book on the phenomenon six years later.
No one expects to see a gorilla walk into a group of people throwing basketballs at each other. (In a similar experiment, Chabris and Simons replaced the gorilla suit with a person carrying an umbrella - inattentional blindness still occurred.) So many people, while focused on a task, don’t.
This experiment shows that sometimes, we literally can’t see things that are right in front of us. Throughout their book, Chabris and Simons discuss the flip side of this. We believe that we see the people passing the basketball.
Inattentional blindness is slightly different than change blindness , which is a type of "blindness" that occurs when we fail to see changes in our environment.
Our memories can be tricky, especially when asked to recall them. We may also believe that we saw or experienced things that have never actually occurred. Both situations can play a role in, for example, the justice system.
Can Multitasking Cause Inattentional Blindness?
Not really; it's closer to the opposite! A Reddit user recently posted that people with ADHD are more likely to see the gorilla, supporting the idea that "focus" is what "blinds" people from the gorilla.
Kenny Conley and Inattentional Blindness
During their research, Chabris and Simons met with Kenny Conley. Conley was a member of the Boston Police Department in 1995. One night, he was chasing a shooting suspect. At the same time, another officer (Michael Cox) was chasing suspects - the officer was undercover, but he was mistaken for one of the suspects. Multiple officers began to assault Cox to the point where Cox was unconscious.
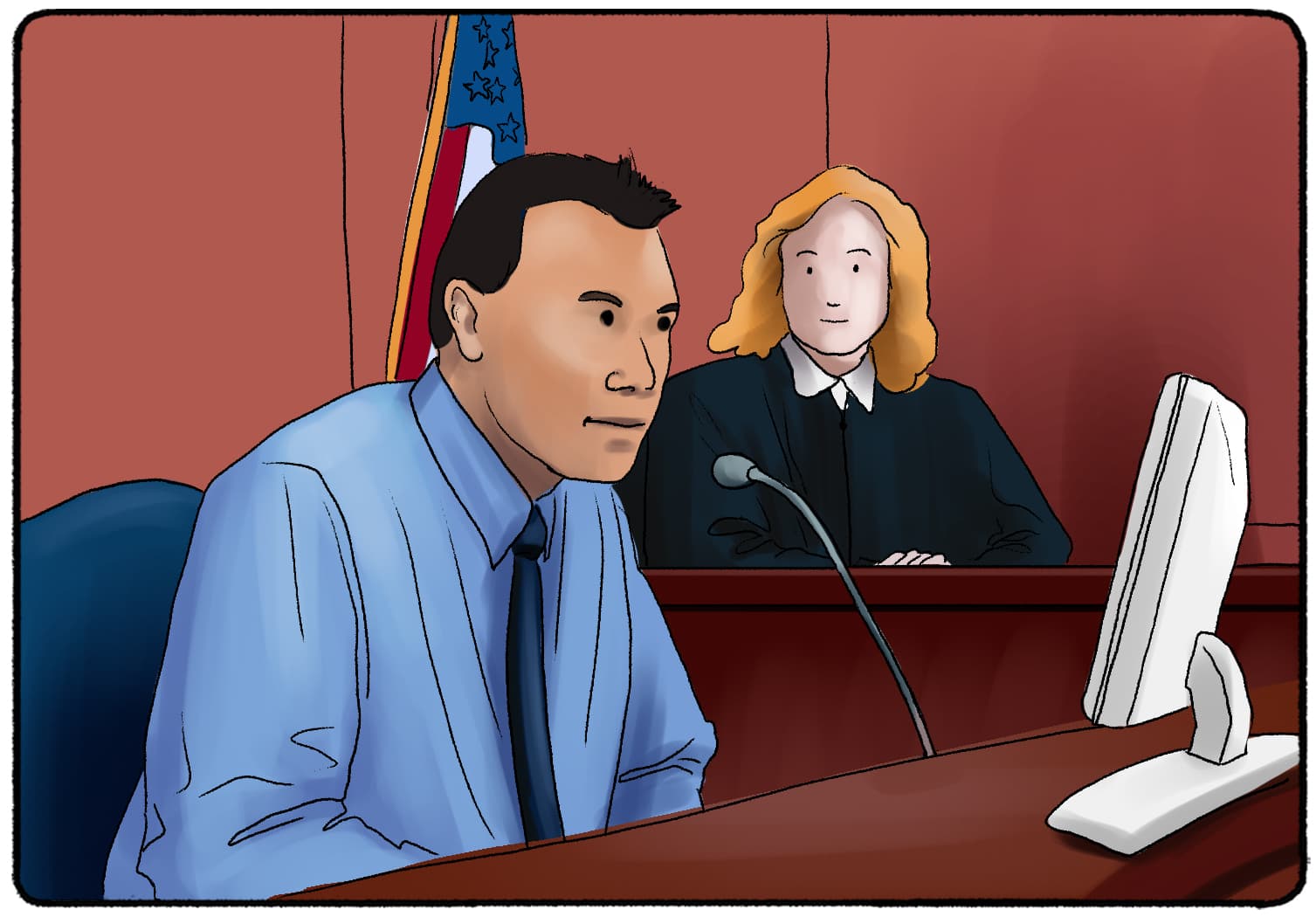
The incident went to trial. Conley was put on the witness stand, for he had been present at the scene of the assault of Michael Cox. But there was just one problem. Conley swore he did not see the assault happen. In his testimony, he says, “I think I would have seen that.” (Sounds much like what people would say after failing to see the gorilla, right?)
Law enforcement officers believed that Conley was lying on the stand. While the officers who assaulted Cox walked free, Conley was charged and put in jail for obstruction of justice and perjury.
But Conley wasn’t lying. He was just so focused on chasing the shooting suspect that he was blind to the assault of Michael Cox. It took ten years of appealing the conviction for Conley to walk free. He was eventually reinstated to his position at the Boston Police Department and received compensation for lost wages.
But Conley’s case is not the only known case of inattentional blindness. Research shows that misidentifications and inattentional blindness are the leading cause of wrongful convictions in this country. The Innocence Project claims that seven out of ten convictions overturned with DNA evidence could be attributed to eyewitness misidentifications. Just this year (2019), the Supreme Court overturned the conviction of Curtis Flowers, a man who was accused of killing four people in 1996. Quite a few examples of witness misidentification are present in the history of this case.
Multiple studies have looked specifically at the abilities of eyewitnesses to thefts and other crimes. Turns out, these witnesses are not always reliable . Due to these studies and the conversation about inattentional blindness, multiple states have created policies for juries on how to spot possible witness misidentification and not to rely too heavily on eyewitness testimony.
Inattentional Blindness In Everyday Life
Even if you are not involved in a criminal trial, it’s important to know about inattentional blindness and how it affects our “intuition.” I highly recommend reading The Invisible Gorilla to learn more about inattentional blindness and how it pervades everyday life.
Related posts:
- Inattentional Blindness (Definition + Examples)
- Change Blindness (Definition + Examples)
- Attention (Psychology Theories)
- Dream of Gorilla Meaning (11 Reasons + Interpretation)
- Cognitive Psychology
Reference this article:
About The Author
- Selective Attention Theories
- Invisible Gorilla Experiment
- Cocktail Party Effect
- Stroop Effect
- Multitasking
- Inattentional Blindness
PracticalPie.com is a participant in the Amazon Associates Program. As an Amazon Associate we earn from qualifying purchases.
Follow Us On:
Youtube Facebook Instagram X/Twitter
Psychology Resources
Developmental
Personality
Relationships
Psychologists
Serial Killers
Psychology Tests
Personality Quiz
Memory Test
Depression test
Type A/B Personality Test
© PracticalPsychology. All rights reserved
Privacy Policy | Terms of Use
Object-based attention: A tutorial review
- Published: 07 June 2012
- Volume 74 , pages 784–802, ( 2012 )
Cite this article

- Zhe Chen 1
22k Accesses
152 Citations
10 Altmetric
Explore all metrics
This tutorial provides a selective review of research on object-based deployment of attention. It focuses primarily on behavioral studies with human observers. The tutorial is divided into five sections. It starts with an introduction to object-based attention and a description of the three commonly used experimental paradigms in object-based attention research. These are followed by a review of a variety of manifestations of object effects and the factors that influence object segmentation. The final two sections are devoted to two key issues in object-based research: the mechanisms that give rise to the object effects and the role of space in object-based selection.
Similar content being viewed by others
The effects of visual search efficiency on object-based attention.

Object-Based Attention: Cognitive and Computational Perspectives
Object-based selection is contingent on attentional control settings.
Avoid common mistakes on your manuscript.
Visual perception is necessarily selective. A natural scene typically contains a vast amount of information. However, because of the limited processing capacity of the visual system at any given time, we cannot process everything simultaneously. Given this limitation, it is perhaps not surprising that the factors that influence visual attention and the mechanisms that underlie the unit of selection are among the most studied topics in modern psychology.
Until the early 1980s, it was generally believed that visual attention operated within a spatial reference frame. This view is perhaps best illustrated by the various metaphors that have been used to describe attention, with the most widely accepted ones being spotlight (B. A. Eriksen & Eriksen, 1974 ; Hoffman & Nelson, 1981 ; Posner, 1980 ; Posner, Snyder, & Davidson, 1980 ), zoom-lens (C. W. Eriksen & St. James, 1986 ; LaBerge, 1983 ), and gradients (Downing & Pinker, 1985 ). Although these models of attention differed regarding their conceptions of the flexibility of attentional selection and the spread of attentional resources within a selected region of space, they all emphasized the spatial properties of attention. Attention was believed to select on the basis of space, and all stimuli within the selected region were thought to receive some degree of processing regardless of observers’ behavioral goals.
Although there is little doubt that space plays an extremely important role in visual selection (for reviews, see Cave, in press ; Cave & Bichot, 1999 ; Lamy & Tsal, 2001 ), by the early 1980s, it had become clear that space was not the only reference frame within which attention operated. Because objects often overlap in space in natural scenes and we seem to have little difficulty attending to a specific feature or object among irrelevant distractors, it makes intuitive sense that the unit of attentional selection may also be based on features and objects, in addition to space.
This tutorial focuses on object-based selection. Footnote 1 Although object is a commonly used word in everyday communication, the question of what an object is in visual perception turns out to be rather difficult to answer (Adelson & Bergen, 1991 ; Duncan, 1984 ; Logan, 1996 ; Scholl, 2001 ). This is because what constitutes an object depends not only on the physical properties of a stimulus or a group of stimuli (Baylis & Driver, 1992 ; Kimchi, Yeshurun, & Cohen-Savranzky, 2007 ; Kramer & Jacobson, 1991 ; Kramer & Watson, 1996 ), but also on how we parse an image in accordance with our behavioral goals (Marr, 1982 ). For the purpose of this tutorial, I will follow previous researchers (e.g., Goldsmith, 1998 ; Kimchi et al., 2007 ) and define a perceptual object as the elements in the visual scene organized by one or more Gestalt grouping principles and/or uniform connectedness. Due to space constraints, I will focus my review of object-based attention primarily on behavioral research, with a very selective review of physiological, neuroimaging, and clinical studies when necessary. The tutorial starts with a description of object-based attentional selection and the three commonly used experimental paradigms in object-based attention research. They are followed by a review of the different manifestations of object effects, the factors that influence object-based deployment of attention, and the mechanisms that give rise to the object effects. In the final part of the tutorial, I review the literature on the role of space in object-based selection. For interested readers, an extensive bibliography can be found at the end of this article.
Object-based attentional selection and three commonly used experimental paradigms
Even in the heyday of the space-based view of attention, various researchers noted the effect of objects on selective attention (e.g., Francolini & Egeth, 1980 ; Kahneman & Chajczyk, 1983 ; Kahneman & Henik, 1981 ; Kahneman, Treisman, & Burkell, 1983 ; Neisser & Becklen, 1975 ). Neisser and Becklen reported that people who were required to perform an attention-demanding task concerning one of two superimposed visual scenes could become remarkably unaware of superthreshold events happening in the unattended scene. Kahneman and Henik ( 1981 ) also found that interference from a task-irrelevant feature of a stimulus was much larger when that feature belonged to an attended object, relative to an unattended object, despite the fact that the locations of these objects were unpredictable. Furthermore, when the task was to report as many items in a display as possible, participants tended to jointly report or jointly miss items that were in the same perceptual group. These results led to the proposal that objects affect the distribution of attention and that attending to one aspect of an object facilitates the processing of other aspects of the same object regardless of task relevancy (Kahneman & Chajczyk, 1983 ; Kahneman & Henik, 1981 ).
In 1984, John Duncan published a seminal study, which arguably marked the beginning of a conceptual change regarding the unit of selection in visual attention. In several experiments, Duncan explored the limits of attention by measuring the number of objects that could be selected simultaneously without a cost. Observers saw stimulus displays that consisted of a bar superimposed on a box (see Fig. 1a ). The bar was either dotted or dashed and was tilted to the left or right, and the box was either small or large and had a gap on the left or right side. The task was to make judgments about one or more of the objects’ features. Observers reported one feature on one object (the bar or the box), two features on the same object, or two features on different objects. Relative to making only one judgment, they showed no decrease in accuracy when the second judgment was on the same object. However, their accuracy decreased when the second judgment was on a different object, demonstrating the limits of attending to two objects simultaneously. Since the bar and box overlapped in space, these results are difficult to explain in terms of spatial-based selection. However, they are consistent with the notion that attention selects the internal representation of an object (but see Watt, 1988 , for an alternative interpretation) and that attending to one aspect of an object entails the processing of all the other aspects that belong to the same object. Similar one-object advantages have since been demonstrated in many studies, both when the primary dependent measure was accuracy (e.g., Kramer, Weber, & Watson, 1997 ; Vecera & Farah, 1994 ) and when it was response latencies (e.g., Baylis & Driver, 1993 ; Behrmann, Zemel, & Mozer, 1998 ; Chen, 2000 ).
Sample displays from Duncan ( 1984 ), Egly, Driver, and Rafal ( 1994 ), and Kramer and Jacobson ( 1991 ). a Stimuli adapted from Duncan. The target display consisted of a bar superimposed on a box. The bar was either dotted or dashed and was tilted to the left or right. The box was small or large and had a gap on the left or right side. The task was to make judgment about one or two object features. Relative to making only one judgment, observers showed no decrease in accuracy when the second judgment was on the same object. However, their accuracy decreased when the second judgment was on a different object. b Stimuli adapted from Egly, Driver, and Rafal ( 1994 ). Observers saw displays that consisted of two rectangles. A precue indicated the most likely location of a subsequent target. On valid trials, the target would appear at the cued location. On invalid same-object trials, the target would appear at the uncued end of the cued rectangle. On invalid different-object trials, the target would appear at the uncued, equidistant end of the other rectangle. The location of the target is represented here by V for valid trials, IS for invalid same-object trials, and ID for invalid different-object trials. Responses were faster on valid than on invalid trials and on invalid same-object than on invalid different-object trials. c Stimuli adapted from Kramer and Jacobson ( 1991 ). The target, which was always at the center, was flanked by distractors that indicated either the same response or a different response. In the same-object condition, the three inner vertical lines and the two horizontal lines all had the same color, while the two outer vertical lines had a different color. In the different-object condition, the central vertical line was in one color, while the other lines were in a different color. The figure on the left was an example of a same-object compatible trial. The figure on the right was an example of a different-object incompatible trial. Interference from distractors was greater in the same-object condition than in the different-object condition
In the 3 decades since Duncan’s ( 1984 ) study, there has been an explosion of research on object-based selection (for reviews, see Driver & Baylis, 1998 ; Kanwisher & Driver, 1992 ; Scholl, 2001 ). One study, which was conducted by Egly, Driver, and Rafal ( 1994 ), is of particular significance, for it introduced a paradigm that allowed the investigation of both space- and object-based deployment of attention within the same experiment. This paradigm has since become the most widely used paradigm in object-based attention research. In Egly, Driver, and Rafal, observers saw two rectangles presented side by side (see Fig. 1b ). A spatial cue then appeared at one of the four ends of the rectangles, followed by a target at one of three locations: the cued location on 75 % of the trials (the valid condition), the uncued end of the cued rectangle on 12.5 % of the trials (the invalid same-object condition), and the uncued equidistant end of the other rectangle on the rest of the trials (the invalid different-object condition). The task was to detect the target as quickly as possible. Observers were faster to respond to the target at the cued location than at either of the uncued locations, indicating space-based attentional facilitation. Furthermore, they were also faster in the invalid same-object condition than in the invalid different-object condition. Since the spatial separation between the cue and the subsequent target was held constant in the latter two conditions, the differential reaction times (RTs) observed in these conditions suggest that attention spreads more quickly to other locations within the same object than between different objects (for alternative interpretations, see Lamy & Egeth, 2002 ; Shomstein & Yantis, 2002 ), indicating object-based deployment of attention. Using variants of Egly, Driver, and Rafal’s two-rectangle paradigm, many researchers have replicated these findings. Regardless of whether the task required stimulus detection or identification, the shift of attention was faster within an object than between objects (e.g., Chen, 1998 ; Lavie & Driver, 1996 ; Macquistan, 1997 ; Moore, Yantis, & Vaughan, 1998 ; Pratt & Sekuler, 2001 ).
A third paradigm commonly used in object-based attention research is the flanker interference paradigm (B. A. Eriksen & Eriksen, 1974 ) with object manipulation. In this paradigm, a target is shown at a central location flanked by distractors that indicate either the same response as or a different response from that of the target (see Fig. 1c ). On some trials (the same-object condition), the target and distractors belong to the same object or perceptual group. On the rest of the trials (the different-object condition), they belong to different objects or perceptual groups. Regardless of whether objects are defined on the basis of contours (e.g., Chen & Cave, 2006 ; Richard, Lee, & Vecera, 2008 ; but see Shomstein & Yantis, 2002 ), Gestalt principles of color (e.g., Baylis & Driver, 1992 ; Harms & Bundesen, 1983 ; Kramer & Jacobson, 1991 ), common motion (e.g., Driver & Baylis, 1989 ; but see Berry & Klein, 1993 ; Kramer, Tham, & Yeh, 1991 ), connectedness (e.g., Kramer & Jacobson, 1991 ), or good continuation (e.g., Baylis & Driver, 1992 ), the general finding is that interference from distractors is greater in the same object/perceptual-grouping condition than in the different object/perceptual-grouping condition. In addition, when focal attention is prevented, observers are more likely to wrongly combine features from different objects when these objects are from the same perceptual group than when they are from different perceptual groups (e.g., Baylis, Driver, & McLeod, 1992 ; Prinzmetal & Keysar, 1989 ). Their ability to track independently moving targets in multiple-object tracking tasks (Pylyshyn & Storm, 1988 ) is also impaired when the targets are merged to form objects such as lines, rubber bands, or Necker cubes (e.g., Scholl, Pylyshyn, & Feldman, 2001 ). These results confirm that items that belong together are selected together.
Other manifestations of object effects
In addition to the findings described above and the paradigms that produced them, object-based attention has also been manifested in a variety of other ways via a number of other methods. Kahneman and Treisman ( 1984 ; Kahneman, Triesman, & Gibbs, 1992 ) were among the first to explore object-based attention. Kahneman et al. ( 1992 ) used an object preview paradigm to investigate the relationship between object continuity and the efficiency of visual information processing. A typical trial consisted of a preview display with two or more letters, each in an individual frame, and a target display with a single letter in one of the frames. The task was to report the identity of the target letter. RTs to the target were reliably shorter when the target was a previewed letter that appeared in the same frame (absolute or relative), as compared with a previewed letter that appeared in a different frame. These results provide evidence for an object-specific preview advantage, which occurs when two objects in close spatiotemporal proximity are seen as different states of the same object relative to different objects.
Recently, Kimchi and her colleagues (Kimchi et al., 2007 ; Yeshurun, Kimchi, Sha’shoua, & Carmel, 2009 ) reported that objects were also capable of capturing attention in a stimulus-driven matter by merely being objects. In several experiments, observers saw displays that consisted of multiple elements, a subset of which formed a perceptual unit (object) on some trials (object trials) and no perceptual unit on the other trials (no-object trials). The task was to report the color of a target, which was defined by its location relative to a cue (see Fig. 2 ). RTs to the target on the object trials were shorter when the cue appeared within the object and longer when the cue occurred outside the object. Because the object was not task relevant or associated with any abrupt onset (and was therefore free of luminance or motion transients), these results provide strong evidence for a unique property of objecthood: It can attract attention in a stimulus-driven manner even though the object has nothing to do with an observer’s behavioral goals. They are also consistent with the findings in prior research that all else being equal, searching for a new object is more efficient than searching for an old object (e.g., Yantis & Hillstrom, 1994 ; Yantis & Jonides, 1996 ; but see also Franconeri, Hollingworth, & Simons, 2005 ).
Sample displays from Kimchi, Yeshurun, and Cohen-Savransky ( 2007 ). In the target display, a subset of the L-shaped elements formed a perceptual unit on some trials (see A and B) and no perceptual units on the other trials (see C). The target was specified in relation to the asterisk by an instruction word (e.g., above , below , left , or right ) at the beginning of each trial. The task was to report the color of the target. The asterisk was inside the perceptual unit in the inside-object condition and outside the perceptual unit in the outside-object condition. There was no perceptual unit in the no-object condition
Several studies have explored the effect of object-based attention on saccadic eye movements. It was found that observers were more likely to make within-object, relative to between-object, eye movements when saccades were required for target identification (e.g., McCarley, Kramer, & Peterson, 2002 ; Theeuwes & Mathot, 2010 ), that the dwell time preceding the saccades was shorter when the switch of attention was within rather than between objects (e.g., McCarley et al., 2002 ), and that in memory recall tasks, participants’ eyes were more likely to fixate on a location when that location was linked, rather than not linked, to an animated creature that presented the relevant information (e.g., Hoover & Richardson, 2008 ).
Object-based attention also enhances manipulations in working memory. In Bao, Li, and Zhang ( 2007 ), participants were required to perform two tasks concurrently: to continuously monitor and update a target’s location on the basis of incoming information and to count the number of times a second stimulus occurred. One group of observers (the separate group) were simply told to perform the two tasks, while the other group (the binding group) was encouraged to integrate the location and object occurrence information into a single object by imagining that the target was a digit 0, which moved to a different location in accordance with incoming location information and which increased its value by 1 every time a second stimulus, whose frequency required monitoring, appeared. The results showed that RTs were longer in the separate group than in the binding group. Moreover, the cost of shifting attention between the location and object occurrence tasks was larger for the separate group than for the binding group, suggesting that binding information to a single object facilitates information manipulations in working memory. Related results were reported by Kahneman and Henik ( 1977 , 1981 ), who manipulated the perceptual groupings of the stimuli that the participants had to recall and found a higher recall rate when the stimuli were displayed in the same perceptual group rather than in different perceptual groups. Ohyama and Watanabe ( 2010 ) observed object-based attentional benefits in memory recognition tasks. Their participants had better recognition memory for letters whose onset coincided with, rather than mismatched, a sudden change that occurred to an object upon which the letters were shown. These results suggest the existence of an object-based attentional mechanism that underlies both scene perception and information retrieval. Attention to one part of an object appears not only to facilitate the speed of information manipulation pertaining to the attended object, but also to enhance the strength of encoding, resulting in better retrieval of the encoded information.
Object-based attention also influences the efficiency of visual search. In general, search efficiency increases with increasing similarity among the distractors and decreasing similarity between the target and the distractors. This perceptual grouping effect has been found with a variety of features, including color, shape, proximity, good continuation, connectedness, and even perceived surface in 3-D space (e.g., Banks & Prinzmetal, 1976 ; Donnelly, Humphreys, & Riddoch, 1991 ; Duncan & Humphreys, 1989 , 1992 ; Z. J. He & Nakayama, 1995 ; Humphreys, Quinlan, & Riddoch, 1989 ; Treisman, 1982 ; Wolfe & Bennett, 1997 ). These results are presumably caused by the fact that, whereas the homogeneity of distractors promotes perceptual grouping, which in turn facilitates their rejection as a perceptual unit, the homogeneity between the target and distractors impairs segmentation, making it harder to distinguish the target from the distractors (Duncan & Humphreys, 1989 , 1992 ). Thus, a line segment was easy to detect when it appeared in isolation but was difficult to detect when it was embedded in a configuration (e.g., Rensink & Enns, 1995 ). Similarly, visual statistical learning—that is, acquiring information about the frequency of stimulus pairing over successive trials—was easier when an attended stimulus was connected with the other (unattended) member of the pair than when the two stimuli were separated (e.g., Baker, Olson, & Behrmann, 2004 ). Searching for two features was also more efficient when the target features belonged to a single object or perceptual group rather than to two different objects or perceptual groups (e.g., Goldsmith, 1998 ; Kahneman & Henik, 1981 ; Wolfe & Bennett, 1997 ). Finally, all else being equal, when a target and a probe differed in orientation, search was more efficient when the target was shown in its canonical orientation rather than in other orientations (e.g., Newell, Brown, & Findlay, 2004 ). These results indicate that object-based attention contributes to both scene perception and information retrieval in long-term memory.
Interestingly, object-based attention has also been found to influence some phenomena that are typically associated with low-level visual processing. Spivey and Spirn ( 2000 ) found that observers who viewed two colored gratings that overlapped in space but differed in orientation could selectively adapt to one of the gratings via attention, resulting in a tilt aftereffect in the direction opposite to the attended grating. Using a different paradigm, Mitchell, Stoner, and Reynolds ( 2004 ) demonstrated the effect of attention on dominance in binocular rivalry. They showed observers two patterns of dots that rotated in opposite directions. The patterns were projected to both eyes. After attention was cued to one pattern, the image of the cued pattern was removed from one eye while the image of the uncued pattern was removed from the other eye. Since the two eyes were now viewing different images, binocular rivalry occurred. Interestingly, although the dominant pattern shifted between the two eyes, as one would expect during binocular rivalry, it was more likely to be the cued pattern, rather than the uncued pattern. In subsequent experiments, Chong and Blake ( 2006 ) showed that in order to counteract the attentional effect of a cued grating on initial dominance in binocularly rivalry, the contrast of the grating had to be reduced by an amount in the neighborhood of 0.3 log-units. Taken together, these findings are consistent with the notion that attention can enhance the early representation of the selected item or its region (Desimone & Duncan, 1995 ), a topic that I will discuss in more detail later.
Although the majority of the literature on object-based attention demonstrates object-based facilitation, object-based inhibition has also been explored. In a typical experiment that uses the inhibition of return (IOR) paradigm (Posner & Cohen, 1984 ), a peripheral location is cued, followed by a central fixation and then a target at either the cued location or a new location. Target detection is facilitated at the cued location when the cue-to-target stimulus onset asynchrony is short (e.g., within 300 msec). However, when it is long (e.g., beyond 300 msec), responses to the target are slower at the cued location relative to an uncued location, demonstrating location-based IOR. It has been proposed that the function of IOR is to prevent repeated sampling of locations that have already been searched (Klein, 1988 ).
Using dynamic displays with moving objects, a number of studies found object-based IOR (e.g., Chou & Yeh, 2008 ; Gibson & Egeth, 1994 ; Jordan & Tipper, 1998 , 1999 ; List & Robertson, 2007 ; Tipper, Driver, & Weaver, 1991 ; Tipper, Weaver, Jerreat, & Burak, 1994 ). Tipper et al. ( 1991 ) cued attention to a moving object and found that IOR moved with the object to a new location, rather than remaining at the original environmental location. Gibson and Egeth ( 1994 ) showed their participants a computer-generated brick that rotated in 3-D and found both location- and object-based IOR. Relative to a control condition in which a cue and a subsequent target appeared at different locations on two different surfaces of the rotating brick, their participants were slower when the cue and target were on different surfaces but at the same environmental location (showing location-based IOR) and when the cue and target appeared on the same surface but at different environmental locations (showing object-based IOR). Similar results were reported in experiments using static displays (e.g., Chou & Yeh, 2008 ; Jordan & Tipper, 1999 ; List & Robertson, 2007 ).
In addition to object-based IOR, object-based inhibition has been demonstrated in the negative priming paradigm. Negative priming refers to the longer RTs to a target on a probe trial (trial n + 1) when that target was a distractor rather than a neutral stimulus on a prime trial (trial n ) (Tipper, 1985 ). In Tipper, Brehaut, and Driver ( 1990 ), participants saw stimulus displays that induced the perception of a target and distractor moving through occluding columns (i.e., the movement itself was never in view), with the target emerging a moment later at either the projected location of the distractor or a different location. Negative priming was found when the target on the probe trial emerged at the projected location of the distractor, even though this location was not the environmental location where the distractor was last seen. In other words, inhibition of the distractor did not simply stay at its original location. Instead, it moved with the inhibited object to its new location, despite the fact that the actual movement of the distractor was never seen. Interestingly, negative priming can be eliminated and even become positive priming when the target and distractor are perceptually grouped in the prime display (e.g., Fuentes, Humphreys, Agis, Carmona, & Catena, 1998 ). Taken together, these results are consistent with the notion of object-based inhibition, suggesting that both facilitation and inhibition can spread across an object’s surface and move with an attended object to its new location.
Object-based attention is not restricted to neurologically intact people. Patients with brain damage have also shown evidence of using an object-based reference frame in visual processing. Driver and Halligan ( 1991 ) showed pairs of vertically aligned nonsense shapes to their patient, P.P., who suffered from severe left neglect due to damage in her right temporo-parietal region. The task was to determine whether the pair of shapes, which were centrally presented, were the same or different. Since neglect is primarily a space-based attentional deficit (Bisiach & Luzzatti, 1978 ), it was not surprising that P.P. performed the task poorly when the shapes differed on the left. Interestingly, when the shapes were tilted 45° to the right, she continued to show poor performance when the shapes differed on their left side even though the differences were now in her intact right side of space. Similar results were reported by a number of other researchers (e.g., Behrmann & Moscovitch, 1994 ; Caramazza & Hillis, 1990 ; Driver, Baylis, & Rafal, 1992 ; Marshall & Halligan, 1994 ; Young, Hellawell, & Welch, 1992 ). In all these studies, patients with neglect in their left visual field were less impaired in performance when the critical information was on the right side of the objects, even when the right side of the objects was in their impaired left side of space (cf. Farah, Brunn, Wong, Wallace, & Carpenter, 1990 ).
A similar pattern of performance can be found in patients with visual extinction, which is a less severe form of neglect confined to a contralesional stimulus when it is presented concurrently with an ipsilesional stimulus. It has been shown that patients with visual extinction can reduce their deficits when the contralesional stimulus is perceptually grouped with the ipsilesional stimulus (e.g., Mattingley, Davis, & Driver, 1997 ; Ward, Goodrich, & Driver, 1994 ). Grouping also improves the perceptual impairments of patients with Balint’s syndrome, who typically see only one object at a time. Humphreys and Riddoch ( 1993 ) tested two Balint’s patients, whose performance in perceiving multiple objects improved remarkably when different-colored objects were connected by black lines. Other object properties also appear to influence the extent of deficits in brain-damaged patients. Humphreys and colleagues (Humphreys & Riddoch, 2003 ; Humphreys, Romani, Olson, Riddoch, & Duncan, 1994 ) found that their patients, who had parietal lobe damage, showed differential degrees of extinction as a function of object type. For example, when pairs of stimuli were shown simultaneously, extinction was more likely to occur with an open geometric shape rather than a closed geometric shape. Remarkably, these patients were often unable to locate the stimulus they had just successfully identified. As Humphreys and his colleagues noted (Humphreys & Riddoch, 2003 ; Humphreys et al., 1994 ), these results suggest that when spatial selection was impaired, the grouping strength between the components of an object could influence the probability of an object being selected, with the object-based selection system favoring the object having the stronger grouping. Moreover, the finding that damage in the parietal lobe could impair the explicit representation of space while leaving the implicit coding of location intact suggests that multiple forms of spatial representation exist in the brain, and not all of them can be accessed explicitly.

Factors that influence object-based selection
Most studies modeled after Egly, Driver, and Rafal ( 1994 ) have used exogenous (peripheral) instead of endogenous (central) cues to direct attention to a specific location in an object. In general, object effects are more readily demonstrated with exogenous than with endogenous cues. Macquistan ( 1997 ) used Egly, Driver, and Rafal’s two-rectangular paradigm but showed one group of participants an exogenous cue and another group an endogenous cue before the onset of the target. Object effects were found with exogenous but not endogenous cues. Similar results were reported by Dagenbach and colleagues (Arrington, Dagenbach, McCartan, & Carr, 2000, November ; Dagenbach, Goolsby, Neely, & Dudziak, 1997 ; Neely & Dagenbach, 1996 ). These findings led some researchers to question whether endogenous control of object-based attention was possible (e.g., Lauwereyns, 1998 ; Macquistan, 1997 ).
However, later research has shown that it is not the type of cue but, rather, the different extent of attentional focus elicited by a cue that determines the presence or absence of an object effect. Goldsmith and Yeari ( 2003 ) noted that because exogenous cues are typically situated peripherally and endogenous cues centrally, participants are more likely to adopt a broad attentional focus with the former and a narrow attentional focus with the latter (see Fig. 3 ). Since a narrow (central) attentional focus presumably weakens the object representation when the objects are relatively large or peripherally located, object effects are more elusive with endogenous than with exogenous cues. Goldsmith and Yeari went on to show that when participants were induced to adopt a broad attentional focus through either task demand or explicit instruction, object effects could be found with endogenous cues. Consistent with this extent-of-attentional-focus account, object effects were found in several other studies that used endogenous cues (e.g., Abrams & Law, 2000 ; Chen & Cave, 2008 ; Law & Abrams, 2002 ). In addition, object effects were more reliable when the task encouraged a wide rather than a narrow deployment of attention (e.g., Lavie & Driver, 1996 ; Shomstein & Yantis, 2002 ; but see Lamy, 2000 , for failure to replicate the finding of Lavie & Driver, 1996 ) and when shifts of attention were required (e.g., Brown & Denney, 2007 ; Lamy & Egeth, 2002 ; Shomstein & Yantis, 2002 ).
The extent of attentional focus, which is shown within the dotted circle, on a typical trial when an exogenous cue is used and when an endogenous cue is used (adapted from Goldsmith & Yeari, 2003 )
For object-based attention to be deployed, a robust object-based representation must be established. Thus, variables that affect the quality of object-based representations also influence the degree to which object-based attention is utilized. One such variable is stimulus presentation duration. Object effects are less reliably elicited with short, relative to long, display durations (e.g., Avrahami, 1999 ; Chen & Cave, 2008 ; Law & Abrams, 2002 ). In Chen and Cave ( 2008 ), participants demonstrated object effects when they had 1,005 ms to view a stimulus display before the appearance of a precue. No object effects were found when the viewing time was decreased to 120 ms. Similar effects of display duration were reported by Avrahami, who manipulated the cue-to-target stimulus onset asynchrony (420 vs. 210 ms), and by Law and Abrams ( 2002 ), who varied the target display duration across experiments (186 vs. 129 ms). In both cases, object effects were more evident with the long, rather than the short, display duration. However, object effects have also been found with display durations as brief as 50 ms (e.g., Duncan, 1984 ). Given the diverse durations that have elicited object effects, it seems that the exact stimulus presentation duration may not really matter. Instead, what matters is the quality of object-based representation that a specific duration allows the participants to establish, which can be influenced by a variety of factors, including task demand, stimulus characteristics, and response mode. Consistent with this idea is the finding by Ariga, Yokosawa, and Ogawa ( 2007 ), who used a modified version of Egly, Driver, and Rafal’s ( 1994 ) two-rectangle paradigm and found no evidence of object-based attention when their participants were not consciously aware of the presented objects (but see Mitroff & Scholl, 2005 , for evidence of forming and updating object representations when changes were made to unseen stimuli during motion-induced blindness).
Another factor that contributes to the quality of object-based representation is the “goodness” of an object. All else being equal, a “good” object is one that has surface uniformity and closed boundaries. Thus, object effects are more reliable when objects show uniform connectedness—for example, when objects have the same color and luminance, as compared with various colors or luminance (e.g., Hecht & Vecera, 2007 ; Kramer & Watson, 1996 ; Matsukura & Vecera, 2006 ; Watson & Kramer, 1999 ), when they have closed rather than open boundaries (e.g., Marino & Scholl, 2005 ), and when targets appear on the same straight line within an object, rather than on different segments of an object separated by angles (e.g., Crundall, Cole, & Galpin, 2007 ).
Object effects are also more robust when the perceptual load is low rather than high (e.g., Ho & Atchley, 2009 ), when the observers are young rather than old (e.g., McCrae & Abrams, 2001 ), when the motor responses required are grasping rather than pointing (e.g., Fischer & Hoellen, 2004 ; Linnell, Humphreys, McIntyre, Laitinen, & Wing, 2005 ; but see Bekkering & Pratt, 2004 , for object-based effect with pointing), and when the left rather than the right hemisphere receives object-related information (e.g., Egly, Driver, & Rafal, 1994 ; Egly, Rafal, Driver, & Starreveld, 1994 ).
As with display duration, factors that promote the “goodness” of an object are conducive to the deployment of object-based attention, but they are not a necessary condition. Object effects have been obtained in objects without closed boundaries (e.g., Avrahami, 1999 , Crundall et al., 2007 ; Kramer & Jacobson, 1991 ) or uniform surfaces (e.g., Hecht & Vecera, 2007 ). Moreover, it has been found in objects created through illusory contours (e.g., Moore et al., 1998 ) and amodal completion (e.g., Behrmann et al., 1998 ; Matsukura & Vecera, 2006 ; Moore et al., 1998 ; Pratt & Sekuler, 2001 ; but see also Saiki, 2000 , for an alternative interpretation of Behrmann et al., 1998 ). These results suggest that the formation of an object representation, regardless of the manner through which such a representation is established, is a critical factor in the deployment of object-based attention.
Support for the statement above is perhaps best found in experiments showing that object-based attention can appear or disappear via the manipulation of an observer’s subjective organization of a stimulus configuration. In several experiments, Chen ( 1998 ) showed her observers displays that resembled two colored V s that were partly superimposed at the base (see Fig. 4 ). When the stimulus configuration was described as two V s, observers were faster at switching attention between the two arms of the same V , as compared with two arms of different V s, demonstrating object-based attention. However, when the same configuration was described as an X made of two different colors, the effect was eliminated. Similar findings were reported by Li and Logan ( 2008 ) and Albrecht, List, and Robertson ( 2008 ). In Li and Logan, skilled Chinese readers showed object effects when they switched attention between Chinese characters that were part of a word, relative to parts of two words. In Albrecht et al., object effects were found when regions were perceived as foreground objects, but not when they were perceived as part of the background. Since objects were defined in these studies by top-down processing, such as subjective organization or the semantic relationship between different stimuli while the physical features of the stimuli were held constant, these results provided unambiguous evidence supporting the notion that the most critical factor in eliciting the deployment of object-based attention is the establishment of a viable object representation.
Stimuli adapted from Chen ( 1998 ). The same stimulus configuration was described as two V s superimposed at the base to some observers but as an X made of two different colors to the other observers. When the configuration was perceived as two V s, an object effect was found. Switching attention within a V was faster than switching attention between two V s. However, when the configuration was perceived as an X , there was no significant difference in RT when the switch of attention was within the same color region or between two different color regions. The location of the target is represented here by V for valid trials, IS for invalid same-object trials, and ID for invalid different-object trials. The arrow indicates a precue
Mechanisms that give rise to object effects
There are three main interpretations regarding the mechanisms that give rise to object effects: sensory enhancement, attentional prioritization, and attentional shifting. The sensory enhancement interpretation emphasizes the spread of attention that respects object boundaries and attributes object effects to the improved sensory representation of the selected object (e.g., Avrahami, 1999 ; Chen & Cave, 2006 , 2008 ; X. He, Fan, Zhou, & Chen, 2004 ; Martínez, Teder-Sälejärvi, & Hillyard, 2007 ; Richard et al., 2008 ; Roelfsema & Houtkamp, 2011 ; Roelfsema, Lamme, & Spekreijse, 1998 ; Valdes-Sosa, Bobes, Rodriguez, & Pinilla, 1998 , Vecera & Farah, 1994 ; Weber, Kramer, & Miller, 1997 ). The attentional prioritization account (as originally presented) stresses the biasing of attentional scanning order in visual search, which, by default, starts from the locations within an already attended object (e.g., Shomstein & Yantis, 2002 , 2004 ). Finally, the attentional shifting account emphasizes the relatively higher cost of attentional shifts between objects, relative to within an object (e.g., Brown & Denney, 2007 ; Lamy & Egeth, 2002 ), and attributes this between-object cost to the additional disengagement operations when attention needs to be disengaged from an object to a location outside that object (Brown & Denney, 2007 ).
When the object-based attentional effect was first reported, it was explained in terms of selecting either the internal representation of the region of space occupied by an attended object (e.g., Kim & Cave, 1995 , 2001 ; Kramer et al., 1997 ) or the internal representation of a location-independent object (e.g., Vecera & Farah, 1994 ). Vecera and Farah referred to these two types of selection as grouped-array and spatially invariant representations, respectively. In both cases, it is assumed that the spread of attention respects object boundaries and that attention improves the quality of the perceptual representation of the selected item. The attentional enhancement is likely to be the result of biased competition (Desimone & Duncan, 1995 ) among neural representations of multiple objects, causing the representation of the attended object to become more effective in its competition with the representations of the other, unattended objects. The selection of the attended object, in turn, leads to faster and/or more accurate processing of the features or items within the object, as compared with those in the nonselected objects.
Early evidence supporting the sensory enhancement account can be found in experiments using single-cell recordings (e.g., Roelfsema et al., 1998 ; Wannig, Rodríguex, & Freiwald, 2007 ). Roelfsema et al. ( 1998 ) measured the responses of neurons in V1 while monkeys were performing a curve-tracing task (see Fig. 5 ). The monkeys were shown two curves on each trial: a target curve, which was directly connected to a fixation point, and a distractor curve, which was not connected with the fixation. On each trial, the monkeys made a saccade to a small circle at the end of the target curve. The results showed that neurons in V1 responded more vigorously when their receptive fields were on the target curve, as compared with the distractor curve. Moreover, this enhancement occurred in neurons whose receptive fields were on different segments of the target curve, relative to different segments of the distractor curve, regardless of whether the two curves were spatially separated or crossed each other. Recent experiments further revealed that the onset of the enhancement of the neurons whose receptive fields were on the target curve differed as a function of the spatial distance between their receptive fields and the fixation, with the onset delayed for those neurons whose receptive fields were farther away from the fixation (Roelfesema & Houtkamp, 2011 ; cf. Roelfsema et al., 1998 ). These results support the notion that attention spreads within an object. They are also consistent with the performance of human observers in mental curve tracing tasks (e.g., Houtkamp, Spekreijse, & Hoelfsema, 2003 ), showing that the spread of attention is a gradual process that takes time to complete.
Sample stimuli and data adapted from Roelfsema and Houtkamp ( 2011 ). a Monkeys were trained to perform a curve-tracing task by making a saccade to the target upon a signal. The target was the circle connected to the fixation point. RF1 and RF2 represent the recording sites for the neurons in V1 whose receptive fields were located on different segments of the target or distractor curve. b Responses of neurons whose receptive fields were in RF1 and RF2. Note that the neurons responded more vigorously when their receptive fields were on the target curve, as compared with the distractor curve. Furthermore, the onset of the enhancement of the neurons whose receptive fields were farther away from the fixation was delayed, relative to that of the neurons whose receptive fields were closer to the fixation
In addition to neurons in V1, the target enhancement effect has also been reported with motion-sensitive neurons in the middle temporal area (MT) of monkeys. Wannig et al. ( 2007 ) cued monkeys to attend to one of two transparent random-dot surfaces and found that the motion of the attended surface activated the neurons in MT more strongly than the motion of the unattended surface, even though the two surfaces occupied the same spatial region. These results provide a direct link between attention to an object or surface and increased neural activation of the representations of the selected object or surface in early sensory areas.
Changes in neuronal responses have also been observed in experiments using event-related brain potentials (ERPs). Valdes-Sosa et al. ( 1998 ) showed their observers stimulus displays consisting of two sets of superimposed dots that differed either in both color and the direction of motion, thus creating the perception of two transparent surfaces in rigid rotation (the two-object condition), or in color but not in the direction of motion, thus creating the perception of one object either at rest or rotating in the same direction (the one-object condition). The participants judged the direction of motion of a subset of the dots (defined by color) that simultaneously underwent brief linear displacements (i.e., nonrotational motion). Their motion-onset ERPs were recorded while the target dots changed locations. Motion-onset posterior P1 and N1 components were found to be associated with both the attended and the unattended sets of dots in the one-object condition, but with only the attended set of dots in the two-object condition. In the latter case, a strong suppression of P1 and N1 was observed with the unattended object. These findings are consistent with the notion that object effects are the result of changes in the neural representations of the selected object. They also suggest that both the enhancement of the attended object and the suppression of the unattended object may play a role in the observed object effects. Results in support of the sensory enhancement account can also be found in a number of other ERP experiments, including X. He et al. ( 2004 , 2008 ), Martínez et al. ( 2007 ; Martínez et al., 2006 ), and Weber et al. ( 1997 ). Despite the differences in their methodology (e.g., using exogenous or endogenous cues or a postdisplay probe to measure the distribution of spatial attention), a common finding is that object-based attention is associated with an enhanced N1 component over the occipito-temporal areas (but see Weber et al., 1997 , for a larger N1 amplitude in the different-object condition than in the same-object condition).
Experiments using functional magnetic resonance imaging (fMRI) have provided converging evidence in support of the sensory enhancement account (e.g., Arrington, Carr, Mayer, & Rao, 2000 ; Martínez, et al., 2006 ; Müller & Kleinschmidt, 2003 ; O’Craven, Downing, & Kanwisher, 1999 ). O’Craven et al. showed their participants semitransparent images of a face and a house that were spatially superimposed. On each trial, either the face or the house would move while the other remained stationery. The participants attended to the face, the house, or the motion in different conditions. The results showed that attention to one attribute (e.g., the face) led to an enhanced blood oxygenation level dependent (BOLD) signal change not only in the brain area associated with the processing of that attribute (i.e., the fusiform face area, which is involved in the processing of faces), but also in the brain area associated with the processing of the task-irrelevant attribute (i.e., the MT/MS area for motion) that belonged to the attended object rather than the unattended object. The finding that neural activation pertaining to a task-irrelevant attribute differs as a function of whether that attribute was part of an attended or an unattended object supports the notion that attention leads to enhanced neural representations of all the attributes that belong to the selected object regardless of task relevancy. Arrington, Carr, et al. ( 2000 ) further showed that attending to a region of space bounded by an object evoked stronger brain activity, as compared with attending to an empty space not bounded by any object. This result indicates that object-based spatial selection requires additional mental resources over and beyond location-based spatial selection. It should be noted, however, that the results above do not entail that the degree of enhanced activation is equivalent in all the regions of the selected object. In fact, Müller and Kleinschmidt ( 2003 ), whose study I will describe in more detail in the next section, found a larger increase in BOLD signal activation at the cued location than at uncued locations in early visual areas (V1–V4). A similar finding was reported by Martínez et al. ( 2006 ) in an ERP experiment where they observed a smaller N1 amplitude associated with object-based attention than with space-based attention.
Shomstein and Yantis ( 2002 ) noted that many experiments that demonstrated object effects required observers to shift attention from one location to another within a trial (e.g., Chen, 1998 ; Egly, Driver, & Rafal, 1994 ; Moore et al., 1998 ). If the default scanning in visual search is to start from locations within an already attended object, this would result in the uncued locations of the attended object being searched before any locations of the unattended object, and this, in turn, would lead to reduced RTs and/or increased accuracy when the target appears in the same object, relative to a different object. In other words, object effects can be the result of attentional prioritization in visual search, rather than the result of attentional spread that respects object boundaries.
To test this hypothesis, Shomstein and Yantis ( 2002 ) manipulated the spatial uncertainty of a target across experiments. The rationale was the following: If the critical factor in triggering object-based attention was scanning order in visual search, knowing the location of the target in advance should eliminate the need for search, resulting in no object effects. The participants saw stimulus displays that resembled a cross: a large rectangle in one orientation (either horizontal or vertical) flanked by a pair of small rectangles in an orthogonal orientation (see Fig. 6 ). On each trial, a target and two distractor letters, which indicated either the same response or different responses, would appear within the boundaries of the configuration. On some trials, all the letters were within the same rectangle. On the other trials, the target was on one rectangle, and the distractors were on different rectangles. In four experiments (Experiments 1–4), the target always appeared at the center, so there was no need to shift attention to locate the target. Although flanker interference effects (B. A. Eriksen & Eriksen, 1974 ) were found, i.e., RTs were shorter when the target and distractors indicated the same response relative to different responses, there was no evidence of an object effect. That is, the magnitude of the interference effects was comparable regardless of whether the target and distractors were on the same object or on different objects. In contrast, when the location of the target was made unpredictable in a subsequent experiment so that observers had to search for the target, an object effect was found, as indicated by larger flanker interference when the target and distractors were on the same object than when they were on different objects. In later studies, Shomstein and colleagues (Shomstein & Yantis, 2004 ; Shomstein & Behrmann, 2008 ) showed that even when the location of a target was unpredictable, object effects could be eliminated when the probability of a target’s appearing on a different object was substantially higher (e.g., 47 %) than that of a target’s appearing at a different location of the cued object (e.g., 7 %). These results were interpreted as evidence that the order of visual search is a critical factor in the manifestation of object effects, in line with the attentional prioritization account (Shomstein & Behrmann, 2008 ; Shomstein & Yantis, 2004 ).
Sample stimuli adapted from Experiments 1–4 of Shomstein and Yantis ( 2002 ). The target display consisted of a target letter and two distractor letters. The target was always the central letter, and the distractors indicated either the same response as the target or a different response from the target. The target and distractors were on the same object in the same-object condition and on different objects in the different-object condition. The magnitude of the distractor interference effects was comparable in the same- and different-object conditions, indicating no object effects
A key prediction of the attentional prioritization account is that object-based attention would not be deployed when the location of a target is known in advance, since the positional certainty of the target would eliminate the need for search and would result in the target location being allocated the highest attentional priority. Although this prediction was confirmed in Shomstein and Yantis ( 2002 ), other studies have shown object effects when the location of the target was known in advance (e.g., Chen & Cave, 2006 , 2008 ; Harms & Bundesen, 1983 ; Kim & Cave, 2001 ; Kramer & Jacobson, 1991 ; Richard et al., 2008 ). Chen and Cave ( 2006 ) used an experimental paradigm similar to that used in Shomstein and Yantis ( 2002 , Experiments 1–4), where the target always appeared at the center of a cross-like configuration. While no object effect was found when participants saw the full cross-like configuration on every trial, it was observed when that configuration appeared on only some of the trials, with the rest of the trials consisting of displays that showed only one or two of the three rectangles. These results are inconsistent with the attentional prioritization account. Chen and Cave ( 2006 ) suggested that mixing the partial displays with the full displays prompted the participants to perceive the stimulus pattern as separate objects, rather than as a single configuration (e.g., a cross). Since subjective organization of a stimulus pattern is known to affect the deployment of object-based attention (e.g., Albrecht et al., 2008 ; Chen, 1998 ; Li & Logan, 2008 ), these results suggest that the key factor in the lack of an object effect in Shomstein and Yantis ( 2002 ) may be the perceived structure of the stimulus configuration, rather than the lack of need for visual search.
Object effects with positional certainty have also been found in Harms and Bundesen ( 1983 ), Kim and Cave ( 2001 ), and Kramer and Jacobson ( 1991 ). These studies all showed that grouping influenced the allocation of attention despite the fact that the target appeared at a known location on every trial. In addition, the observers in Chen and Cave ( 2008 ) responded faster to letters located at the two ends of a single object, relative to two ends of different objects, even though in both cases the onset of the targets was preceded by an endogenous central cue of 100 % validity. Richard et al. ( 2008 ) used a flanker interference paradigm with a centrally located target and found object effects when the target was a part of an object (i.e., belonged to the object), but not when it was a letter sitting on top of a rectangle. On the basis of their results, Richard et al. proposed that the key factor in obtaining object-based attention under the condition of positional certainty was the perception of the task-relevant feature as an integral part of an object shape, rather than as something perceptually segregated from the object shape. It should be pointed out, however, that this interpretation did not explain why object effects were observed in other studies where the task-relevant feature was clearly not an integral part of an object shape (e.g., Chen & Cave, 2006 , 2008 ). Regardless of what induced the object effects found in Richard et al., the finding of an object effect even when the location of the target was known in advance suggests that object effects are not just a by-product of the order in which different regions of a scene are visited during visual search.
To date, the strongest physiological evidence supporting the within-group spread of attention has come from several recent studies by Roelfsema and colleagues (e.g., Roelfsema & Houtkamp, 2011 ; Wannig, Stanisor, & Roelfsema, 2011 ). In one experiment by Wannig et al. ( 2011 ), monkeys were shown displays that consisted of two target bars and two distractor bars. The task was to fixate on a fixation point, to wait for a dot to appear at one of the target bars, and upon the offset of the dot, to make a saccade to the indicated target bar. The researchers simultaneously recorded the responses of V1 neurons from two sites: site 1 for those neurons whose receptive field was on one of the target bars, and site 2 for those neurons whose receptive field was on one of the distractor bars. The results show that the appearance of the dot in the receptive field of site 1 triggered not only an increase in activity in those neurons whose receptive field was in site 1, but also an increase in those neurons whose receptive field was in site 2. Furthermore, the activity of the neurons was stronger when site 2 was on a distractor bar collinear to the target bar (i.e., in the same perceptual group), rather than when the two bars were not aligned collinearly (i.e., in different perceptual groups). Similar results were observed for perceptual groupings based on color or common fate. Since site 2 was on a distractor bar, these results provided direct evidence that attention could spread to task-irrelevant stimuli outside the focus of attention and that the attentional enhancement was greater when these stimuli were bound to the attended stimulus through one or more Gestalt grouping principles.
More recently, Drummond and Shomstein ( 2010 ) suggested that in addition to search order, attentional prioritization can also be the result of a parallel search process where information at different locations of a configuration is extracted at different rates according to attentional priority and that attentional prioritization can affect the quality of the sensory representation of an attended object. In this revised model, there is little difference between the attentional prioritization account and the sensory enhancement account.
As was mentioned earlier, object effects have also been explained in terms of the relative cost of shifting attention within an object versus between objects (Brown & Denney, 2007 ; Lamy & Egeth, 2002 ). Lamy and Egeth used a variant of Egly, Driver, and Rafal’s ( 1994 ) two-rectangle paradigm and asked their participants to perform tasks that either required or did not require shifts of attention. Object effects were found in the former but not in the latter. For example, the participants demonstrated an object effect when the task was to detect the presence of a target, and its onset was preceded by a precue indicating the most likely location of the target. In contrast, there was no evidence of an object effect when the task was to judge the size of two simultaneously presented targets whose onsets were not preceded by a precue. Lamy and Egeth interpreted these results in the context of required attentional shifts within a trial (cf. Drummond & Shomstein, 2010 ). Whereas the precue in the detection task encouraged the participants to switch attention from the cued location to the target location, the simultaneous onset of a pair of targets with no precue in the size judgment task induced the participants to adopt a diffuse attentional window without the need to switch attention. Since shifting attention between objects is more difficult and, therefore, has a higher cost than shifting attention within an object, object effects are typically found in cuing paradigms where the location of the target is uncertain and attentional shifts are required within a trial.
Building on Lamy and Egeth’s ( 2002 ) results, Brown and Denney ( 2007 ) investigated the role of the individual component of attention that contributed to the cost in the between-object shift of attention. According to Posner and his colleagues (e.g., Posner & Cohen, 1984 ; Posner & Peterson, 1990 ), the process of shifting attention consists of three separate operations: disengagement (i.e., the release of attention from a current location), movement (i.e., the switching of attention from one location to another), and engagement (i.e., the focusing of attention to a new location). To investigate the disengagement and engagement operations, Brown and Denney designed a series of tasks that required participants to switch attention in a variety of ways (see Fig. 7 ). In addition to performing tasks in a two-rectangle display that involved shifting attention within an object or between two objects (the two-invalid-within condition and the two-invalid-between condition, respectively), the participants also saw one-rectangle displays that required them to shift attention between two locations inside the same rectangle (the one-invalid-within condition), two locations outside the rectangle (the one-location-to-location condition), from a location inside the rectangle to a location outside (the one-object-to-location condition), and from a location outside the rectangle to a location inside (the one-location-to-object condition), among others. Two main results were found. First, disengaging attention from an object was associated with an additional cost over and above that of disengaging attention from a location or shifting attention within an object. This was indicated by the longer RTs in the object-to-location condition than in the location-to-object condition, location-to-location condition, and invalid-within-object condition. Second, engaging attention to an object after attentional movement did not necessarily incur an extra cost, relative to engaging attention to a location. This was evidenced by comparable RTs between the object-to-location condition and the two-object-invalid-between condition. On the basis of these results, Brown and Denney proposed that shifting attention from a location and from an object may involve different or separate processes (see also Arrington, Carr, et al., 2000 ) and that object effects reflect primarily the cost of disengagement operations associated with the object-based attention. It should be noted, however, that other researchers have shown object effects under situations where attentional shifts are not required (e.g., Chen & Cave, 2006 ; Duncan, 1984 ; Harms & Bundesen, 1983 ; Kim & Cave, 2001 ; Kramer & Jacobson, 1991 ; Richard et al., 2008 ), suggesting that the cost in between-object attentional shifts may be one of several factors that contribute to object effects.
Sample stimuli adapted from Brown and Denney ( 2007 ). a and b Conditions in two-object displays. c – f Conditions in one-object displays
Taken together, the available evidence suggests that there is substantial flexibility in how attention is distributed within an object and how fully a stimulus configuration is segregated into objects. Many factors, including those reviewed in the previous section (e.g., attentional focus, “goodness” of an object, etc.) and the probability of the target’s appearing within a cued versus an uncued object, all influence the deployment of object-based attention. Attention spreads within an object, yet the spreading of attention is not necessarily automatic. Furthermore, although object segregation may often be triggered spontaneously, it is not an automatic process. Object segregation and/or object-based allocation of attention as a result of object segregation can all be subject to strategic control (Yeari & Goldsmith, 2010 ).
The role of space in object-based selection
Although many studies have shown that location plays a special role in selective attention (e.g., Cave & Pashler, 1995 ; Chen, 2009 ; Kim & Cave, 1995 ; Tsal & Lavie, 1993 ; for reviews, see also Cave, in press ; Lamy & Tsal, 2001 ), the role of space in object-based selection is not straightforward. Whereas some studies have reported results consistent with object-based attention selecting a location-independent representation, where attention selects the features of an attended object, such as its shape, color, orientation, and texture, without selecting its spatial location (e.g., Awh, Dhaliwal, Christensen, & Matsukura, 2001 ; Matsukura & Vecera, 2011 ; O’Craven et al., 1999 ; Vecera & Farah, 1994 ), other studies have found that object-based attention selects from a location-mediated representation, where attention selects the regions of space occupied by the attended object (e.g., Arrington, Carr, et al., 2000 ; Kim & Cave, 1995 ; Kramer et al., 1997 ; Martínez et al., 2007 ; Martínez et al., 2006 ; Müller & Kleinschmidt, 2003 ; Valdes-Sosa et al., 1998 ; Vecera & Farah, 1994 ; Weber et al., 1997 ). As was mentioned earlier, Vecera and Farah (see also Vecera, 1994 ) referred to these two types of selection as spatially invariant and grouped-array selection, respectively.
The first study to distinguish between these two types of selection was conducted by Vecera and Farah ( 1994 ), who used a variant of Duncan’s ( 1984 ) bar-on-box paradigm. Participants saw displays that consisted of a bar and a box that were either superimposed at fixation (the superimposed condition) or positioned in separate spatial locations on the left or right of fixation (the separated condition). The task was to report two features that belonged to the same object or to different objects. Vecera and Farah reasoned that selection from a location-invariant representation would result in an object effect of comparable magnitude from both the superimposed and separated conditions. In contrast, selection from a location-mediated representation would lead to a larger object effect in the separated condition than in the superimposed condition. Implicit in this reasoning was the assumption that the cost of switching attention between objects would increase with their spatial separation (see Kramer et al., 1997 , for arguments against this assumption; but see also Vecera, 1997 , for counterarguments). The results showed that the object effects were comparable in the superimposed and separated conditions. Moreover, in a subsequent experiment where the task was stimulus detection instead of feature identification, a larger object effect was observed in the separated condition than in the superimposed condition. On the basis of these results, Vecera and Farah concluded that object-based attention could select from both location-independent and location-mediated representations and that the level of selection in a specific task depended on the nature of the representations required by the task.
Kramer et al. ( 1997 ) later challenged these conclusions. In two experiments, they measured observers’ object-based deployment of attention and their distribution of spatial attention within the same paradigm. Observers saw a bar and a box that were either superimposed or separated in space. To hold visual acuity constant across the two conditions, the bar and the box in the superimposed condition were displayed on the left or right side of fixation, with a filler on the other side. In addition to reporting object features that were part of the same or different objects, the observers, on a small number of trials, were also required to detect the presence of a small probe when it appeared immediately after the offset of the object display. These postdisplay probe trials were included to measure observers’ distribution of spatial attention (Kim & Cave, 1995 ). A larger object effect was found in the feature identification task in the separated condition, as compared with the superimposed condition. Moreover, RT to the probe was shorter when it appeared at the location of the object that possessed both of the target features, rather than at the location of the object that possessed neither of the target features. These results suggest that the location of the attended object was selected even when the task was feature identification. Importantly, a similar probe RT result was observed in a subsequent experiment, where Kramer et al. ( 1997 ) placed the objects in the superimposed condition at fovea, as in Vecera and Farah’s ( 1994 ) original study, and replicated the latter’s results of comparable object effects in the superimposed and separated object conditions. Taken together, these results support the notion that object-based attention is accompanied by the selection of the internal representation of an object’s location.
Several other studies have reported findings consistent with a location-mediated selection of object-based attention. Using a paradigm that involved moving objects, Lamy and Tsal ( 2000 ) found attentional effects both at the old location of a precue (i.e., the cued location of an object before it started to move) and at the new location that followed the moving object. Similarly, O’Grady and Müller ( 2000 ) reported increased target detectability at all the locations along the contour of a cued object, relative to an uncued object. Müller and Kleinschmidt ( 2003 ) measured their participants’ BOLD signals during a gap discrimination task in an fMRI study. The participants saw displays that consisted of wrench-like objects. A central cue, which was several seconds long, indicated the most likely location of the target. As in a typical experiment on object-based attention, the target could appear at the cued location (the valid condition) or at an uncued location either on the cued object (the invalid same-object condition) or on the other object (the invalid different-object condition). Both space and object effects were found in RTs. Moreover, participants showed an increase in BOLD signal activation in response to the cue in early visual cortical areas (V1–V4) at the retinotopic representations of not only the cued location relative to the uncued locations, but also the uncued location of the same object relative to that of a different object. These results were in line with the findings of Roelfsema and colleagues (Roelfsema et al., 1998 ; Wannig et al., 2011 ), who showed object-based modulations of neuronal responses in V1. The fact that object-based attention modulated neural activation in the early visual areas provides evidence that attending to an object entails the selection of that object’s location.
A similar conclusion was reached by Weber et al. ( 1997 ) in an ERP study. Observers saw two partially overlapping objects on either the left or right side of fixation. The task was to judge whether a prespecified color/shape conjunction was present in the display. The task-relevant features, if present, were on either a single object (the same-object condition) or two different objects (the different-object condition). On some trials, a task-irrelevant small probe would appear after the offset of the target display. These probe trials did not require overt responses. However, the participants’ ERPs in response to the onset of the probe were measured. The results most relevant here were the findings from the probe trials. When the probe appeared at the location previously occupied by objects that contained the target features, a larger P1 was found in the same-object than in the different-object condition. Since P1 is known to indicate the distribution of spatial attention (e.g., Hillyard et al., 1996 ; Luck, Heinze, Mangun, & Hillyard, 1990 ), this result, together with the results from other ERP studies (e.g., Martínez et al., 2007 ; Martínez et al., 2006 ) and fMRI and single-cell recording studies (e.g., Müller & Kleinschmidt, 2003 ; Roelfsema et al., 1998 ), provides physiological evidence supporting the location-mediated selection of object-based attention.
Matsukura and Vecera ( 2011 ) recently proposed that a spatially invariant representation could occur under conditions when objects were clearly segregated. They showed participants displays that consisted of a bar superimposed on a box. Object effects were found when attention could be directed to a specific object or objects in advance (e.g., when participants knew one or both of the to-be-reported features before the onset of the object display), but not when the knowledge of the to-be-reported features was withheld until after the offset of the object display. However, when the bar and the box were shown in different colors at separate spatial locations, object effects were observed in the absence of advance knowledge of the to-be-reported features when the objects were in view. Furthermore, the magnitude of the object effect was not influenced by the extent of spatial separation between the objects (2.48° vs. 5.24°). On the basis of these results, Matsukura and Vecera concluded that object-based attention could select from space-invariant representations so long as the objects in question could be easily individuated. However, caution should be taken in interpreting these results, for there is evidence that spatial attention does not necessarily shift in an analog fashion (e.g., C. W. Eriksen & Murphy, 1987 ; Yantis, 1988 ). Perhaps the role of space in object-based selection is best illustrated in a recent study by Hollingworth, Maxcey-Richard, and Vecera ( 2012 ), who found interaction between space- and object-based attention within the same experimental paradigm. Consistent with the notion that there are linkages between lower-level spatial representations and higher-level spatially invariant representations at multiple levels of selection (Di Lollo, Enns, & Rensink, 2000 ; Hochstein & Ahissar, 2002 ; Roelfsema & Houtkamp, 2011 ; van der Velde & de Kamps, 2001 ), Hollingworth et al. showed that whereas spatial attention forms a gradient across an attended object, the spread of this gradient is constrained by the boundaries of the object.
Since Duncan’s ( 1984 ) seminal study, many advances have been made regarding the mechanisms that underlie the selection of visual attention. It is now generally accepted that attention selects the internal representation of both space and object, that space- and object-based attention interact, and that they are often evoked within the same visual scene. Object-based attention is frequently but not mandatorily deployed, and there are many factors that influence object segmentation. When object-based attention is deployed, it typically acts via the selection of an object’s location, resulting in enhanced quality of the sensory representation of the selected object and more efficient processing of the features that belong to that object. It is important to recognize that although this tutorial emphasizes object-based selection, attention can also select features and surfaces in addition to space. Our visual system uses different types of attention to give us a unified view of the world.
Due to space constraints, the literature on feature-based attention is not included in this review. Feature-based attention refers to the enhanced sensitivity to a feature value (e.g., a specific orientation, color, or motion direction) similar to an attended feature value regardless of whether the former is at the attended location or belongs to the attended object (see Maunsell & Treue, 2006 , for a review). For example, Treue and Martínez-Trujillo ( 1999 ; Martínez-Trujillo & Treue, 2004 ) showed that attending to a specific motion direction at one location enhanced the gain of MT neurons selective to the attended direction even though the receptive fields of the affected neurons were in the opposite visual hemifield. In addition to motion, feature-based attention has been found in several other feature dimensions, including spatial frequency, orientation, and color, and the attentional effects have been demonstrated in both physiological and psychophysical studies (e.g., Arman, Ciaramitaro, & Boynton, 2006 ; Liu, Larsson, & Carrasco, 2007 ; Roelfsema, Khayat, & Spekreijse, 2003 ; Rossi & Paradiso, 1995 ; Sàenz, Buračas, & Boynton, 2002 , 2003 ; Shulman & Wilson, 1987 ; White & Carrasco, 2011 ). Although feature-based attentional effects can contribute to object-based effects and vice versa under certain experimental conditions, these two types of effects can be dissociated (see Wannig et al., 2011 ). Whereas feature-based attentional effects are not limited to a perceptual object or group, object-based attentional effects are confined to the attended object or perceptual group.
Abrams, R. A., & Law, M. B. (2000). Object-based visual attention with endogenous orienting. Perception & Psychophysics, 62, 818–833.
Article Google Scholar
Adelson, E. H., & Bergen, J. R. (1991). The plenoptic function and the elements of early vision. In M. Landy & J. A. Movshon (Eds.), Computational models of visual processing (pp. 3–20). Cambridge, MA: MIT Press.
Google Scholar
Albrecht, A. R., List, A., & Robertson, L. C. (2008). Attentional selection and the representation of holes and objects. Journal of Vision, 8, 1–10.
Article PubMed Google Scholar
Ariga, A., Yokosawa, K., & Ogawa, H. (2007). Object-based attentional selection and awareness of objects. Visual Cognition, 15, 685–709.
Arman, A. C., Ciaramitaro, V. M., & Boynton, G. M. (2006). Effects of feature-based attention on the motion aftereffect at remote locations. Vision Research, 46, 2968–2976.
Arrington, C. M., Carr, T. H., Mayer, A. R., & Rao, S. M. (2000). Neural mechanisms of visual attention: Object-based selection of a region in space. Journal of Cognitive Neuroscience, 12, 106–117.
Arrington, C. M., Dagenbach, D., McCartan, M. K., & Carr, T. H. (2000, November). The reliability of object-based attention following peripheral and central cues . Poster presented at the 41st Annual Meeting of the Psychonomic Society, New Orleans, LA.
Avrahami, J. (1999). Objects of attention, objects of perception. Perception & Psychophysics, 61, 1604–1612.
Awh, E., Dhaliwal, H., Christensen, S., & Matsukura, M. (2001). Evidence for two components of object-based attention. Psychological Science, 12, 329–334.
Baker, C. I., Olson, C. R., & Behrmann, M. (2004). Role of attention and perceptual grouping in visual statistical learning. Psychological Science, 15, 460–466.
Banks, W. P., & Prinzmetal, W. (1976). Configurational effects in visual information processing. Perception & Psychophysics, 19, 361–367.
Bao, M., Li, Z.-H., & Zhang, D.-R. (2007). Binding facilitates attention switching within working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33, 959–969.
Baylis, G. C., & Driver, J. (1992). Visual parsing and response competition: The effect of grouping factors. Perception & Psychophysics, 51, 145–162.
Baylis, G. C., & Driver, J. (1993). Visual attention and objects: Evidence for hierarchical coding of location. Journal of Experimental Psychology. Human Perception and Performance, 19, 451–470.
Baylis, G. C., Driver, J., & McLeod, P. (1992). Movement and proximity constrain miscombinations of colour and form. Perception, 21, 201–218.
Behrmann, M., & Moscovitch, M. (1994). Object-centered neglect in patients with unilateral neglect: Effects of left–right coordinates of objects. Journal of Cognitive Neuroscience, 6, 1–16.
Behrmann, M., Zemel, R. S., & Mozer, M. C. (1998). Object-based attention and occlusion: Evidence from normal participants and a computational model. Journal of Experimental Psychology. Human Perception and Performance, 24, 1011–1036.
Bekkering, H., & Pratt, J. (2004). Object-based processes in the planning of goal-directed hand movements. Quarterly Journal of Experimental Psychology, 57A, 1345–1368.
Berry, G., & Klein, R. (1993). Does motion-induced grouping modulate the flanker compatibility effect? A failure to replicate Driver & Baylis. Canadian Journal of Experimental Psychology, 47, 714–729.
PubMed Google Scholar
Bisiach, E., & Luzzatti, C. (1978). Unilateral neglect of representational space. Cortex, 14, 129–133.
Brown, J. M., & Denney, H. I. (2007). Shifting attention into and out of objects: Evaluating the processes underlying the object advantage. Perception & Psychophysics, 69, 606–618.
Caramazza, A., & Hillis, A. E. (1990). Internal spatial representation of written words: Evidence from unilateral neglect. Nature, 346, 267–279.
Cave, K. R. (in press). Spatial attention. In D. Reisberg (Ed.), Oxford handbook of cognitive psychology . New York: Oxford University Press.
Cave, K. R., & Bichot, N. P. (1999). Visuospatial attention: Beyond a spotlight model. Psychonomic Bulletin & Review, 6, 204–223.
Cave, K. R., & Pashler, H. (1995). Visual selection mediated by location: Selecting successive visual objects. Perception & Psychophysics, 57, 421–432.
Chen, Z. (1998). Switching attention within and between objects: The role of subjective organization. Canadian Journal of Experimental Psychology, 52, 7–16.
Chen, Z. (2000). An object-based cost of visual filtering. Perception & Psychophysics, 62, 482–495.
Chen, Z. (2009). Not all features are created equal: Processing asymmetries between location and object features. Vision Research, 49, 1481–1491.
Chen, Z., & Cave, K. R. (2006). Reinstating object-based attention under positional certainty: The importance of subjective parsing. Perception & Psychophysics, 68, 992–1003.
Chen, Z., & Cave, K. R. (2008). Object-based attention with endogenous cuing and positional certainty. Perception & Psychophysics, 70, 1435–1443.
Chong, S. C., & Blake, R. (2006). Exogenous attention and endogenous attention influence initial dominance in binocular rivalry. Vision Research, 46, 1794–1803.
Chou, W.-L., & Yeh, S.-L. (2008). Location- and object-based inhibition of return are affected by different kinds of working memory. Quarterly Journal of Experimental Psychology, 61, 1761–1768.
Crundall, D., Cole, G. G., & Galpin, A. (2007). Object-based attention is mediated by collinearity of targets. Quarterly Journal of Experimental Psychology, 60, 137–153.
Dagenbach, D., Goolsby, B., Neely, C. A., & Dadziak, K. M. (1997, November). Further studies of attention to space and objects with endogenous cueing . Poster presented at the 38th Annual Meeting of the Psychonomic Society, Philadelphia, PA.
Desimone, R., & Duncan, J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222.
Di Lollo, V., Enns, J. T., & Rensink, R. (2000). Competition for consciousness among visual events: The psychophysics of reentrant visual processes. Journal of Experimental Psychology. General, 129, 481–507.
Donnelly, N., Humphreys, G. W., & Riddoch, M. J. (1991). Parallel computation of primitive shape descriptions. Journal of Experimental Psychology. Human Perception and Performance, 17, 561–570.
Downing, C. J., & Pinker, S. (1985). The spatial structure of visual attention. In M. I. Posner & O. S. M. Marin (Eds.), Attention and performance XI (pp. 171–187). Hillsdale, NJ: Erlbaum.
Driver, J., & Baylis, G. C. (1989). Movement and visual attention: The spotlight metaphor breaks down. Journal of Experimental Psychology. Human Perception and Performance, 15, 448–456.
Driver, J., & Baylis, G. C. (1998). Attention and visual object segmentation. In R. Parasuraman (Ed.), The attentive brain (pp. 299–325). Cambridge, MA: MIT Press.
Driver, J., Baylis, G. C., & Rafal, R. D. (1992). Preserved figure–ground segregation and symmetry perception in visual neglect. Nature, 360, 73–75.
Driver, J., & Halligan, P. W. (1991). Can visual neglect operate in object-centred co-ordinates? An affirmative single-case study. Cognitive Neuropsychology, 8, 475–496.
Drummond, L., & Shomstein, S. (2010). Object-based attention: Shifting or uncertainty? Attention, Perception, & Psychophysics, 72, 1743–1755.
Duncan, J. (1984). Selective attention and the organization of visual information. Journal of Experimental Psychology. General, 123, 501–517.
Duncan, J., & Humphreys, G. (1989). Visual search and stimulus similarity. Psychological Review, 96, 433–458.
Duncan, J., & Humphreys, G. (1992). Beyond the search surface: Visual search and attentional engagement. Journal of Experimental Psychology. Human Perception and Performance, 18, 578–588.
Egly, R., Driver, J., & Rafal, R. D. (1994). Shifting visual attention between objects and locations: Evidence from normal and parietal lesion subjects. Journal of Experimental Psychology. General, 123, 161–177.
Egly, R., Rafal, R., Driver, J., & Starreveld, Y. (1994). Hemispheric specialization for object-based attention in a split brain patient. Psychological Science, 5, 380–383.
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of target letter in a nonsearch task. Perception & Psychophysics, 16, 143–149.
Eriksen, C. W., & Murphy, T. D. (1987). Movement of attentional focus across the visual field: A critical look at the evidence. Perception & Psychophysics, 42, 299–305.
Eriksen, C. W., & St. James, J. (1986). Visual attention within and around the field of focal attention: A zoom lens model. Perception & Psychophysics, 40, 225–240.
Farah, M. J., Brunn, J. L., Wong, A. B., Wallace, M. A., & Carpenter, P. (1990). Frames of reference for allocating attention to space: Evidence from the neglect syndrome. Neuropsychologia, 28, 335–347.
Fischer, M. H., & Hoellen, N. (2004). Space- and object-based attention depend on motor intention. The Journal of General Psychology, 131, 365–377.
Francolini, C. M., & Egeth, H. A. (1980). On the nonautomaticity of “automatic” activation: Evidence of selective seeing. Perception & Psychophysics, 27, 331–342.
Franconeri, S. L., Hollingworth, A., & Simons, D. J. (2005). Do new objects capture attention? Psychological Science, 16, 275–281.
Fuentes, L. J., Humphreys, G. W., Agis, I. F., Carmona, E., & Catena, A. (1998). Object-based perceptual grouping affects negative priming. Journal of Experimental Psychology. Human Perception and Performance, 24, 664–672.
Gibson, B. S., & Egeth, H. (1994). Inhibition of return to object-based and environment-based locations. Perception & Psychophysics, 55, 323–339.
Goldsmith, M. (1998). What’s in a location? Comparing object-based and space-based models of feature integration in visual search. Journal of Experimental Psychology. General, 127, 189–219.
Goldsmith, M., & Yeari, M. (2003). Modulation of object-based attention by spatial focus under endogenous and exogenous orienting. Journal of Experimental Psychology. Human Perception and Performance, 29, 897–918.
Harms, L., & Bundesen, C. (1983). Color segregation and selective attention. Perception & Psychophysics, 33, 11–19.
He, X., Fan, S., Zhou, K., & Chen, L. (2004). Cue validity and object-based attention. Journal of Cognitive Neuroscience, 16, 1085–1097.
He, X., Humphreys, G., Fan, S., Chen, L., & Han, S. (2008). Differentiating spatial and object-based effects on attention: an event-related brain potential study with peripheral cueing. Brain Research, 1245 , 116-125.
He, Z. J., & Nakayama, K. (1995). Visual attention to surfaces in three-dimensional space. Proceedings of the National Academy of Sciences, 92, 11155–11159.
Hecht, L. N., & Vecera, S. P. (2007). Attentional selection of surface uniformity and part structure. Psychonomic Bulletin & Review, 14, 1205–1211.
Hillyard, S. A., Anllo-Vento, L., Clark, V., Henze, H., Luck, S., & Mangun, G. (1996). Neuroimaging approaches to the study of visual attention: A tutorial. In A. F. Kramer, M. G. Coles, & G. D. Logan (Eds.), Converging operations in the study of visual selective attention (pp. 107–138). Washington, DC: APA Press.
Chapter Google Scholar
Ho, M.-C., & Atchley, P. (2009). Perceptual load modulates object-based attention. Journal of Experimental Psychology. Human Perception and Performance, 35, 1661–1669.
Hochstein, S., & Ahissar, M. (2002). View from the top: Hierarchies and reverse hierarchies in the visual system. Neuron, 36, 791–804.
Hoffman, J. E., & Nelson, B. (1981). Spatial selectivity in visual search. Perception & Psychophysics, 30, 283–290.
Hollingworth, A., Maxcey-Richard, A. M., & Vecera, S. P. (2012). The spatial distribution of attention within and across objects. Journal of Experimental Psychology. Human Perception and Performance, 38, 135–151.
Hoover, M. A., & Richardson, D. C. (2008). When facts go down the rabbit hole: Contrasting features and objecthood as indexes to memory. Cognition, 108, 533–542.
Houtkamp, R., Spekreijse, H., & Roelfsema, P. R. (2003). A gradual spread of attention during mental curve tracing. Perception & Psychophysics, 65, 1136–1144.
Humphreys, G. W., Quinlan, P. T., & Riddoch, M. J. (1989). Grouping processes in visual search: Effects with single- and combined-feature targets. Journal of Experimental Psychology. General, 118, 258–279.
Humphreys, G. W., & Riddoch, M. J. (1993). Interactions between object and space-systems revealed through neuropsychology. In D. E. Meyer & S. S. Kornblum (Eds.), Attention and performance XIV (pp. 143–162). Cambridge, MA: MIT Press.
Humphreys, G. W., & Riddoch, M. J. (2003). From what to where: Neuropsychological evidence for implicit interactions between object- and space-based attention. Psychological Science, 14, 487–492.
Humphreys, G. W., Romani, C., Olson, A., Riddoch, M., & Duncan, J. (1994). Nonspatial extinction following lesions of the parietal lobes in humans. Nature, 372, 357–359.
Jordan, H., & Tipper, S. P. (1998). Object-based inhibition of return in static displays. Psychonomic Bulletin & Review, 5, 504–509.
Jordan, H., & Tipper, S. P. (1999). Spread of inhibition across an object’s surface. British Journal of Psychology, 90, 495–507.
Kahneman, D., & Chajczyk, D. (1983). Tests of the automaticity of reading: Dilution of Stroop effects by color-irrelevant stimuli. Journal of Experimental Psychology. Human Perception and Performance, 9, 497–509.
Kahneman, D., & Henik, A. (1977). Effects of visual grouping on immediate recall and selective attention. In S. Dornic (Ed.), Attention and performance VI (pp. 307–332). Hillsdale, NJ: Erlbaum.
Kahneman, D., & Henik, A. (1981). Perceptual organization and attention. In M. Kubovy & J. R. Pomerantz (Eds.), Perceptual organization (pp. 181–211). Hillsdale, NJ: Erlbaum.
Kahneman, D., & Treisman, A. (1984). Changing views of attention and automaticity. In R. Parasuraman & D. A. Davies (Eds.), Varieties of attention (pp. 29–61). New York: Academic Press.
Kahneman, D., Treisman, A., & Burkell, J. (1983). The cost of visual filtering. Journal of Experimental Psychology. Human Perception and Performance, 9, 510–522.
Kahneman, D., Treisman, A., & Gibbs, B. J. (1992). The reviewing of object files: Object-specific integration of information. Cognitive Psychology, 24, 175–219.
Kanwisher, N., & Driver, J. (1992). Objects, attributes, and visual attention: Which, what, and where. Current Directions in Psychological Science, 1, 26–31.
Kim, M.-S., & Cave, K. R. (1995). Spatial attention in visual search for features and feature conjunctions. Psychological Science, 6, 376–380.
Kim, M.-S., & Cave, K. R. (2001). Perceptual grouping via spatial selection in a focused-attention task. Vision Research, 41, 611–624.
Kimchi, R., Yeshurun, Y., & Cohen-Savransky, A. (2007). Automatic, stimulus-driven attentional capture by objecthood. Psychonomic Bulletin & Review, 14, 166–172.
Klein, R. (1988). Inhibitory tagging system facilitates visual search. Nature, 334, 430–431.
Kramer, A. F., & Jacobson, A. (1991). Perceptual organization and focused attention: The role of objects and proximity in visual processing. Perception & Psychophysics, 50, 267–284.
Kramer, A. F., Tham, M. P., & Yeh, Y. Y. (1991). Movement and focused attention: A failure to replicate. Perception & Psychophysics, 50, 537–546.
Kramer, A. F., & Watson, S. E. (1996). Object-based visual selection and the principle of uniform connectedness. In A. F. Kramer, M. G. H. Coles, & G. D. Logan (Eds.), Converging operations in the study of visual selective attention (pp. 395–414). Washington, DC: American Psychological Association.
Kramer, A. F., Weber, T. A., & Watson, S. E. (1997). Object-based attentional selection: Grouped arrays or spatially invariant representations? Comment on Vecera and Farah (1994). Journal of Experimental Psychology. General, 126, 3–13.
LaBerge, D. (1983). Spatial extent of attention to letters in words. Journal of Experimental Psychology. Human Perception and Performance, 9, 371–379.
Lamy, D. (2000). Object-based selection under focused attention: A failure to replicate. Perception & Psychophysics, 62, 1272–1279.
Lamy, D., & Egeth, H. (2002). Object-based selection: The role of attentional shifts. Perception & Psychophysics, 64, 52–66.
Lamy, D., & Tsal, Y. (2000). Object features, object locations, and object files: Which does selective attention activate and when? Journal of Experimental Psychology. Human Perception and Performance, 26, 1387–1400.
Lamy, D., & Tsal, Y. (2001). On the status of location in visual attention. European Journal of Cognitive Psychology, 13, 305–342.
Lauwereyns, J. (1998). Exogenous/endogenous control of space-based/object-based attention: Four types of visual selection. European Journal of Cognitive Psychology, 10, 41–74.
Lavie, N., & Driver, J. (1996). On the spatial extent of attention in object-based visual selection. Perception & Psychophysics, 58, 1238–1251.
Law, M. B., & Abrams, R. A. (2002). Object-based selection with and beyond the focus of spatial attention. Perception & Psychophysics, 64, 1017–1027.
Li, X., & Logan, G. D. (2008). Object-based attention in Chinese readers of Chinese words: Beyond Gestalt principles. Psychonomic Bulletin & Review, 15, 945–949.
Linnell, K. J., Humphreys, G. W., McIntyre, D. B., Laitinen, S., & Wing, A. M. (2005). Action modulates object-based selection. Vision Research, 45, 2268–2286.
List, A., & Robertson, L. C. (2007). Inhibition of return and object-based attentional selection. Journal of Experimental Psychology. Human Perception and Performance, 33, 1322–1334.
Liu, T., Larsson, J., & Carrasco, M. (2007). Feature-based attention modulates orientation-selective responses in human visual cortex. Neuron, 55, 313–323.
Logan, G. (1996). The CODE theory of visual attention: An integration of space-based and object-based attention. Psychological Review, 103, 603–649.
Luck, S. J., Heinze, H. J., Mangun, G. R., & Hillyard, S. A. (1990). Visual event-related potential index of focused attention within bilateral stimulus array: II. Functional dissociation of P1 and N1 components. Electroencephalography & Clinical Neurophysiology, 75, 528–542.
Macquistan, A. (1997). Object based allocation of visual attention in response to exogenous, but not endogenous, spatial precues. Psychonomic Bulletin & Review, 4, 512–515.
Marino, A. C., & Scholl, B. J. (2005). The role of closure in defining the “objects” of object-based attention. Perception & Psychophysics, 67, 1140–1149.
Marr, D. (1982). Vision . New York: Freeman.
Marshall, J. C., & Halligan, P. W. (1994). The yin and yang of visuo-spatial neglect: A case study. Neuropsychologia, 32, 1037–1057.
Martínez, A., Teder-Sälejärvi, W., & Hillyard, S. A. (2007). Spatial attention facilitates selection of illusory objects: Evidence from event-related brain potentials. Brain Research, 1139, 143–152.
Martínez, A., Teder-Sälejärvi, W.,Vazquez, M., Molholm, S., Foxe, J. J., Javitt, D. C., … Hillyard, S. A. (2006). Objects are highlighted by spatial attention. Journal of Cognitive Neuroscience , 18 , 298–310.
Martínez-Trujillo, J. C., & Treue, S. (2004). Feature-based attention increases the selectivity of population responses in primate visual cortex. Current Biology, 14, 744–751.
Matsukura, M., & Vecera, S. P. (2006). The return of object-based attention: Selection of multiple-region objects. Perception & Psychophysics, 68, 1163–1175.
Matsukura, M., & Vecera, S. P. (2011). Object-based selection from spatially-invariant representations: Evidence from a feature-report task. Attention, Perception, & Psychophysics, 73, 447–457.
Mattingley, J. B., Davis, G., & Driver, J. (1997). Preattentive filling-in of visual surface in parietal extinction. Science, 275, 671–674.
Maunsell, J. H. R., & Treue, S. (2006). Feature-based attention in visual cortex. Trends in Neurosciences, 29, 317–322.
McCarley, J., Kramer, A. F., & Peterson, M. S. (2002). Overt and covert object-based attention. Psychonomic Bulletin & Review, 9, 751–758.
McCrae, C. S., & Abrams, R. A. (2001). Age-related differences in object- and location-based inhibition of return of attention. Psychology and Aging, 16, 437–449.
Mitchell, J. F., Stoner, G. R., & Reynolds, J. H. (2004). Object-based attention determines dominance in binocular rivalry. Nature, 429, 410–413.
Mitroff, S. R., & Scholl, B. J. (2005). Forming and updating object representations without awareness: Evidence from motion-induced blindness. Vision Research, 45, 961–967.
Moore, C. M., Yantis, S., & Vaughan, B. (1998). Object-based visual attention: Evidence from perceptual completion. Psychological Science, 9, 104–110.
Müller, N. G., & Kleinschmidt, A. (2003). Dynamic interaction of object- and space-based attention in retinotopic visual areas. Journal of Neuroscience, 23, 9812–9816.
Neely, C. A., & Dagenbach, D. (1996, November). Exogenous and endogenous cuing: Spatial versus object-based visual attention . Poster presented at the 37th Annual Meeting of the Psychonomic Society, Chicago, IL.
Neisser, U., & Becklen, R. (1975). Selective looking: Attending to visually specified events. Cognitive Psychology, 7, 480–494.
Newell, F. N., Brown, V., & Findlay, J. M. (2004). Is object search mediated by object-based or image-based representations? Spatial Vision, 17, 511–541.
O’Craven, K. M., Downing, P. E., & Kanwisher, N. (1999). fMRI evidence for objects as the units of attentional selection. Nature, 401, 584–587.
O’Grady, R. B., & Müller, H. J. (2000). Object-based selection operates on a grouped array of locations. Perception & Psychophysics, 62, 1655–1667.
Ohyama, J., & Watanabe, K. (2010). Exogenous temporal cues enhance recognition memory in an object-based manner. Attention, Perception, & Psychophysics, 72, 2157–2167.
Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32, 3–25.
Posner, M. I., & Cohen, Y. (1984). Components of visual orienting. In H. Bouma & D. G. Bowhuis (Eds.), Attention and performance X (pp. 531–556). Hillsdale, NJ: Erlbaum.
Posner, M. I., & Peterson, S. E. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42.
Posner, M. I., Snyder, C. R. R., & Davidson, B. J. (1980). Attention and the detection of signals. Journal of Experimental Psychology. General, 109, 106–174.
Pratt, J., & Sekuler, A. B. (2001). The effects of occlusion and past experience on the allocation of object-based attention. Psychonomic Bulletin & Review, 8, 721–727.
Prinzmetal, W., & Keysar, B. (1989). Functional theory of illusory conjunctions and neon colors. Journal of Experimental Psychology. General, 118, 165–190.
Pylyshyn, Z. W., & Storm, R. W. (1988). Tracking multiple independent targets: Evidence for a parallel tracking mechanism. Spatial Vision, 3, 179–197.
Rensink, R. A., & Enns, J. T. (1995). Preemption effects in visual search: Evidence for low-level grouping. Psychological Review, 102, 101–130.
Richard, A. M., Lee, H., & Vecera, S. P. (2008). Attentional spreading in object-based attention. Journal of Experimental Psychology. Human Perception and Performance, 34, 842–853.
Roelfsema, P. R., & Houtkamp, R. (2011). Incremental grouping of image elements in vision. Attention, Perception, & Psychophysics, 73, 2542–2572.
Roelfsema, P. R., Khayat, P. S., & Spekreijse, H. (2003). Subtask sequencing in the primary visual cortex. Proceedings of the National Academy of Sciences, 100, 5467–5472.
Roelfsema, P. R., Lamme, V. A. F., & Spekreijse, H. (1998). Object-based attention in the primary visual cortex of the macaque monkey. Nature, 395, 376–381.
Rossi, A. F., & Paradiso, M. A. (1995). Feature-specific effects of selective visual attention. Vision Research, 35, 621–634.
Sàenz, M., Buračas, G. T., & Boynton, G. M. (2002). Global effects of feature-based attention in human visual cortex. Nature Neuroscience, 5, 631–632.
Sàenz, M., Buračas, G. T., & Boynton, G. M. (2003). Global feature-based attention for motion and color. Vision Research, 43, 629–637.
Saiki, J. (2000). Occlusion, symmetry, and object-based attention: Comment on Behrmann, Zemel, and Mozer (1998). Journal of Experimental Psychology. Human Perception and Performance, 26, 424–433.
Scholl, B. J. (2001). Objects and attention: The state of the art. Cognition, 80, 1–46.
Scholl, B. J., Pylyshyn, Z. W., & Feldman, J. (2001). What is a visual object? Evidence from target merging in multiple object tracking. Cognition, 80, 159–177.
Shomstein, S., & Behrmann, M. (2008). Object-based attention: Strength of object representation and attentional guidance. Perception & Psychophysics, 70, 132–144.
Shomstein, S., & Yantis, S. (2002). Object-based attention: Sensory modulation or priority setting? Perception & Psychophysics, 64, 41–51.
Shomstein, S., & Yantis, S. (2004). Configural and contextual prioritization in object-based attention. Psychonomic Bulletin & Review, 11, 247–253.
Shulman, G. L., & Wilson, J. (1987). Spatial frequency and selective attention to local and global information. Perception, 16, 89–101.
Spivey, M. J., & Spirn, M. J. (2000). Selective visual attention modulates the direct tilt aftereffect. Perception & Psychophysics, 62, 1525–1533.
Theeuwes, J., & Mathot, S. (2010). Object-based eye movements: The eyes prefer to stay within the same object. Attention, Perception, & Psychophysics, 72, 597–601.
Tipper, S. P. (1985). The negative priming effect: Inhibitory effects of ignored primes. Quarterly Journal of Experimental Psychology, 37A, 571–590.
Tipper, S. P., Brehaut, J. C., & Driver, J. (1990). Selection of moving and static objects for the control of spatially directed action. Journal of Experimental Psychology. Human Perception and Performance, 16, 492–504.
Tipper, S. P., Driver, J., & Weaver, B. (1991). Object-centred inhibition of return of visual attention. Quarterly Journal of Experimental Psychology, 43A, 289–298.
Tipper, S. P., Weaver, B., Jerreat, L. M., & Burak, A. L. (1994). Object-based and environment-based inhibition of return of visual attention. Journal of Experimental Psychology. Human Perception and Performance, 20, 478–499.
Treisman, A. (1982). Perceptual grouping and attention in visual search for features and for objects. Journal of Experimental Psychology. Human Perception and Performance, 8, 194–214.
Treue, S., & Martínez-Trujillo, J. C. (1999). Feature-based attention influences motion processing gain in macaque visual cortex. Nature, 399, 575–579.
Tsal, Y., & Lavie, N. (1993). Location dominance in attending to color and shape. Journal of Experimental Psychology. Human Perception and Performance, 19, 131–139.
Valdes-Sosa, M., Bobes, M. A., Rodriguez, V., & Pinilla, T. (1998). Switching attention without shifting the spotlight: Object-based attentional modulation of brain potentials. Journal of Cognitive Neuroscience, 10, 137–151.
van der Velde, F., & de Kamps, M. (2001). From knowing what to knowing where: Modelling object-based attention with feedback disinhibition of activation. Journal of Cognitive Neuroscience, 13, 479–491.
Vecera, S. P. (1994). Grouped locations and object-based attention: Comment on Egly, Driver, and Rafal (1994). Journal of Experimental Psychology. General, 123, 316–320.
Vecera, S. P. (1997). Grouped arrays versus object-based representations: Reply to Kramer et al. (1997). Journal of Experimental Psychology: General, 126, 14–18.
Vecera, S. P., & Farah, M. J. (1994). Does visual attention select objects or locations? Journal of Experimental Psychology. General, 123, 146–160.
Wannig, A., Rodríguez, V., & Freiwald, W. A. (2007). Attention to surfaces modulates motion processing in extrastriate area MT. Neuron, 54, 639–651.
Wannig, A., Stanisor, L., & Roelfsema, P. R. (2011). Automatic spread of attentional response modulation along Gestalt criteria in primary visual cortex. Nature Neuroscience, 14, 1243–1244.
Ward, R., Goodrich, S., & Driver, J. (1994). Grouping reduces visual extinction. Visual Cognition, 1, 101–129.
Watson, S. E., & Kramer, A. F. (1999). Object-based visual selective attention and perceptual organization. Perception & Psychophysics, 61, 31–49.
Watt, R. (1988). Visual processing: Computational, psychophysical and cognitive research . London: Erlbaum.
Weber, T. A., Kramer, A. F., & Miller, A. G. (1997). Selective processing of superimposed objects: An electrophysiological analysis of object-based attentional selection. Biological Psychology, 45, 159–182.
White, A. L., & Carrasco, M. (2011). Feature-based attention involuntarily and simultaneously improves visual performance across locations. Journal of Vision, 11, 1–10.
Wolfe, J. M., & Bennett, S. C. (1997). Preattentive object files: Shapeless bundles of basic features. Vision Research, 37, 25–43.
Yantis, S. (1988). On analog movements of attention. Perception & Psychophysics, 43, 203–206.
Yantis, S., & Hillstrom, A. P. (1994). Stimulus-driven attentional capture: Evidence form equiluminant visual objects. Journal of Experimental Psychology. Human Perception and Performance, 20, 95–107.
Yantis, S., & Jonides, J. (1996). Attentional capture by abrupt onsets: New perceptual objects or visual masking. Journal of Experimental Psychology. Human Perception and Performance, 22, 1505–1513.
Yeari, M., & Goldsmith, M. (2010). Is object-based attention mandatory? Strategic control over mode of attention. Journal of Experimental Psychology. Human Perception and Performance, 36, 565–579.
Yeshurun, Y., Kimchi, R., Sha’shoua, G., & Carmel, T. (2009). Perceptual objects capture attention. Vision Research, 49, 1329–1335.
Young, A. W., Hellawell, D. J., & Welch, J. (1992). Neglect and visual recognition. Brain, 115, 51–71.
Download references
Author note
I thank Kyle Cave, Morris Goldsmith, Pieter Roelfsema, and Jeremy Wolfe for their helpful comments on an earlier version of the manuscript.
Author information
Authors and affiliations.
Department of Psychology, University of Canterbury, Private Bag 4800, Christchurch, New Zealand
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zhe Chen .
Rights and permissions
Reprints and permissions
About this article
Chen, Z. Object-based attention: A tutorial review. Atten Percept Psychophys 74 , 784–802 (2012). https://doi.org/10.3758/s13414-012-0322-z
Download citation
Published : 07 June 2012
Issue Date : July 2012
DOI : https://doi.org/10.3758/s13414-012-0322-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Object-based
- Tutorial review
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 03 March 2022
A brain-based general measure of attention
- Kwangsun Yoo ORCID: orcid.org/0000-0002-5213-4575 1 ,
- Monica D. Rosenberg ORCID: orcid.org/0000-0001-6179-4025 1 , 2 ,
- Young Hye Kwon ORCID: orcid.org/0000-0001-7754-4223 1 ,
- Qi Lin ORCID: orcid.org/0000-0001-9702-8584 1 ,
- Emily W. Avery ORCID: orcid.org/0000-0002-8481-3978 1 ,
- Dustin Sheinost ORCID: orcid.org/0000-0002-6301-1167 3 ,
- R. Todd Constable ORCID: orcid.org/0000-0001-5661-9521 3 , 4 , 5 &
- Marvin M. Chun ORCID: orcid.org/0000-0003-1070-7993 1 , 4 , 6 , 7
Nature Human Behaviour volume 6 , pages 782–795 ( 2022 ) Cite this article
5159 Accesses
12 Citations
75 Altmetric
Metrics details
- Network models
Attention is central to many aspects of cognition, but there is no singular neural measure of a person’s overall attentional functioning across tasks. Here, using original data from 92 participants performing three different attention-demanding tasks during functional magnetic resonance imaging, we constructed a suite of whole-brain models that can predict a profile of multiple attentional components (sustained attention, divided attention and tracking, and working memory capacity) for novel individuals. Multiple brain regions across the salience, subcortical and frontoparietal networks drove accurate predictions, supporting a common (general) attention factor across tasks, distinguished from task-specific ones. Furthermore, connectome-to-connectome transformation modelling generated an individual’s task-related connectomes from rest functional magnetic resonance imaging, substantially improving predictive power. Finally, combining the connectome transformation and general attention factor, we built a standardized measure that shows superior generalization across four independent datasets (total N = 495) of various attentional measures, suggesting broad utility for research and clinical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
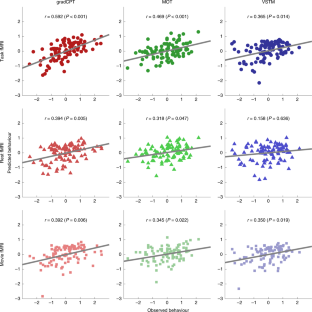
Similar content being viewed by others
The subcortical and neurochemical organization of the ventral and dorsal attention networks
Heterogenous brain activations across individuals localize to a common network
Heritability and interindividual variability of regional structure-function coupling
Data availability.
Raw task and rest fMRI data used in the primary analyses ( n = 92) are available at https://doi.org/10.15154/1520622 .
Code availability
Scripts for the predictive model (the general attention model, C2C model and CPM) construction are available for download at https://github.com/rayksyoo/General_Attention . Scripts for the other (statistical) analyses are available from the corresponding author upon request.
Chun, M. M., Golomb, J. D. & Turk-Browne, N. B. A taxonomy of external and internal attention. Annu. Rev. Psychol. 62 , 73–101 (2011).
Article PubMed Google Scholar
Weissman, D. H., Roberts, K. C., Visscher, K. M. & Woldorff, M. G. The neural bases of momentary lapses in attention. Nat. Neurosci. 9 , 971–978 (2006).
Article CAS PubMed Google Scholar
Heinrichs, R. W. & Zakzanis, K. K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12 , 426–445 (1998).
Biederman, J., Newcorn, J. & Sprich, S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am. J. Psychiatry 148 , 564–577 (1991).
Levin, H. S. et al. Neurobehavioral outcome following minor head injury: a three-center study. J. Neurosurg. 66 , 234–243 (1987).
Rosenberg, M. D. et al. Functional connectivity predicts changes in attention observed across minutes, days, and months. Proc. Natl Acad. Sci. U. S. A. 117 , 3797–3807 (2020).
Article CAS PubMed PubMed Central Google Scholar
Kucyi, A. et al. Prediction of stimulus-independent and task-unrelated thought from functional brain networks. Nat. Commun. 12 , 1793 (2021).
Deary, I. J., Penke, L. & Johnson, W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 11 , 201–211 (2010).
Miyake, A. et al. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: a latent variable analysis. Cogn. Psychol. 41 , 49–100 (2000).
Huang, L., Mo, L. & Li, Y. Measuring the interrelations among multiple paradigms of visual attention: an individual differences approach. J. Exp. Psychol. Hum. Percept. Perform. 38 , 414–428 (2012).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3 , 215–229 (2002).
Article CAS Google Scholar
Kanwisher, N. & Wojciulik, E. Visual attention: insights from brain imaging. Nat. Rev. Neurosci. 1 , 91–100 (2000).
Rosenberg, M. D., Finn, E. S., Scheinost, D., Constable, R. T. & Chun, M. M. Characterizing attention with predictive network models. Trends Cogn. Sci. 21 , 290–302 (2017).
Rosenberg, M. D. et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 19 , 165–171 (2016).
Wu, E. X. W. et al. Overlapping attentional networks yield divergent behavioral predictions across tasks: neuromarkers for diffuse and focused attention? Neuroimage 209 , 116535 (2020).
Kucyi, A., Hove, M. J., Esterman, M., Hutchison, R. M. & Valera, E. M. Dynamic brain network correlates of spontaneous fluctuations in attention. Cereb. Cortex 27 , 1831–1840 (2017).
PubMed Google Scholar
Shen, X. et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 12 , 506–518 (2017).
Finn, E. S. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18 , 1664–1671 (2015).
Woo, C. W., Chang, L. J., Lindquist, M. A. & Wager, T. D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. 20 , 365–377 (2017).
Gratton, C. et al. Defining individual-specific functional neuroanatomy for precision psychiatry. Biol. Psychiatry 88 , 28–39 (2020).
Cohen, J. R. & D’Esposito, M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 36 , 12083–12094 (2016).
Yoo, K. et al. Multivariate approaches improve the reliability and validity of functional connectivity and prediction of individual behaviors. Neuroimage 197 , 212–223 (2019).
Rosenberg, M. D. et al. Methylphenidate modulates functional network connectivity to enhance attention. J. Neurosci. 36 , 9547–9557 (2016).
Rosenberg, M. D., Hsu, W.-T., Scheinost, D., Todd Constable, R. & Chun, M. M. Connectome-based models predict separable components of attention in novel individuals. J. Cogn. Neurosci. 30 , 160–173 (2018).
Yoo, K. et al. Connectome-based predictive modeling of attention: comparing different functional connectivity features and prediction methods across datasets. Neuroimage 167 , 11–22 (2018).
Lin, Q. et al. Resting-state functional connectivity predicts cognitive impairment related to Alzheimer’s disease. Front. Aging Neurosci. 10 , 94 (2018).
Article PubMed PubMed Central Google Scholar
Avery, E. W. et al. Distributed patterns of functional connectivity predict working memory performance in novel healthy and memory-impaired individuals. J. Cogn. Neurosci. 32 , 241–255 (2019).
Zhang, H. et al . Do intrinsic brain functional networks predict working memory from childhood to adulthood? Hum. Brain Mapp . https://doi.org/10.1002/hbm.25143 (2020).
Tomasi, D. & Volkow, N. D. Network connectivity predicts language processing in healthy adults. Hum. Brain Mapp. 41 , 3696–3708 (2020).
Beaty, R. E. et al. Robust prediction of individual creative ability from brain functional connectivity. Proc. Natl Acad. Sci. U. S. A. 115 , 1087–1092 (2018).
Hsu, W.-T., Rosenberg, M. D., Scheinost, D., Constable, R. T. & Chun, M. M. Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc. Cogn. Affect. Neurosci. 13 , 224–232 (2018).
Jiang, R. et al. Connectome-based individualized prediction of temperament trait scores. Neuroimage 183 , 366–374 (2018).
Cai, H., Chen, J., Liu, S., Zhu, J. & Yu, Y. Brain functional connectome-based prediction of individual decision impulsivity. Cortex 125 , 288–298 (2020).
Esterman, M., Noonan, S. K., Rosenberg, M. & Degutis, J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb. Cortex 23 , 2712–2723 (2013).
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I. & Posner, M. I. The activation of attentional networks. Neuroimage 26 , 471–479 (2005).
Kardan, O. et al . Adult neuromarkers of sustained attention and working memory predict inter- and intra-individual differences in these processes in youth. Preprint at bioRxiv https://doi.org/10.1101/2021.08.01.454530 (2021).
Engle, R. W. Working memory capacity as executive attention. Curr. Dir. Psychol. Sci. 11 , 19–23 (2002).
Article Google Scholar
Yoo, K. et al . A cognitive state transformation model for task-general and task-specific subsystems of the brain connectome. Preprint at bioRxiv https://doi.org/10.1101/2020.12.23.424176 (2020).
Noble, S. et al. Influences on the test-retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb. Cortex 27 , 5415–5429 (2017).
Varoquaux, G. Cross-validation failure: small sample sizes lead to large error bars. NeuroImage 180 , 68–77 (2018).
Jangraw, D. C. et al. A functional connectivity-based neuromarker of sustained attention generalizes to predict recall in a reading task. Neuroimage 166 , 99–109 (2018).
Fountain-Zaragoza, S., Samimy, S., Rosenberg, M. D. & Prakash, R. S. Connectome-based models predict attentional control in aging adults. Neuroimage 186 , 1–13 (2019).
Van Essen, D. C. et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 80 , 62–79 (2013).
DuPaul, G. J., Power, T. J., Anastopoulos, A. D. & Reid, R. ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation (Guilford, 1998).
Consortium, T. A.-200. The ADHD-200 Consortium: a model to advance the translational potential of neuroimaging in clinical neuroscience. Front. Syst. Neurosci. 6 , 62 (2012).
Google Scholar
Satterthwaite, T. D. et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. NeuroImage 86 , 544–553 (2014).
Casey, B. J. et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 32 , 43–54 (2018).
Wojciulik, E. & Kanwisher, N. The generality of parietal involvement in visual attention. Neuron 23 , 747–764 (1999).
Duncan, J. & Owen, A. M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23 , 475–483 (2000).
Ramnani, N. & Owen, A. M. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 5 , 184–194 (2004).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24 , 167–202 (2001).
Pardo, J. V., Fox, P. T. & Raichle, M. E. Localization of a human system for sustained attention by positron emission tomography. Nature 349 , 61–64 (1991).
Corbetta, M., Shulman, G. L., Miezin, F. M. & Petersen, S. E. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Sci. (80-.) 270 , 802–805 (1995).
Hopfinger, J. B., Buonocore, M. H. & Mangun, G. R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 3 , 284–291 (2000).
Sprague, T. C. & Serences, J. T. Attention modulates spatial priority maps in the human occipital, parietal and frontal cortices. Nat. Neurosci. 16 , 1879–1887 (2013).
Wimmer, R. D. et al. Thalamic control of sensory selection in divided attention. Nature 526 , 705–709 (2015).
Heinze, H. J. et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 372 , 543–546 (1994).
Coull, J. T., Vidal, F., Nazarian, B. & Macar, F. Functional anatomy of the attentional modulation of time estimation. Sci. (80-.) 303 , 1506–1508 (2004).
Gao, J. H. et al. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Sci. (80-.). 272 , 545–547 (1996).
Leiner, H. C., Leiner, A. L. & Dow, R. S. Does the cerebellum contribute to mental skills? Behav. Neurosci. 100 , 443–454 (1986).
Petersen, S. E., Fox, P. T., Posner, M. I., Mintun, M. & Raichle, M. E. Positron emission tomographic studies of the processing of single words. J. Cogn. Neurosci. 1 , 153–170 (1989).
Stoodley, C. J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11 , 352–365 (2012).
Strick, P. L., Dum, R. P. & Fiez, J. A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 32 , 413–434 (2009).
Buckner, R. L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80 , 807–815 (2013).
Allen, G., Buxton, R. B., Wong, E. C. & Courchesne, E. Attentional activation of the cerebellum independent of motor involvement. Sci. (80-.) 275 , 1940–1943 (1997).
Rees, G., Frackowiak, R. & Frith, C. Two modulatory effects of attention that mediate object categorization in human cortex. Sci. (80-.) 275 , 835–838 (1997).
Gottwald, B., Mihajlovic, Z., Wilde, B. & Mehdorn, H. M. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia 41 , 1452–1460 (2003).
Greene, A. S., Gao, S., Scheinost, D. & Constable, R. T. Task-induced brain state manipulation improves prediction of individual traits. Nat. Commun. 9 , 2807 (2018).
Article PubMed PubMed Central CAS Google Scholar
Jiang, R. et al. Task-induced brain connectivity promotes the detection of individual differences in brain–behavior relationships. Neuroimage 207 , 116370 (2020).
Sui, J., Jiang, R., Bustillo, J. & Calhoun, V. Neuroimaging-based individualized prediction of cognition and behavior for mental disorders and health: methods and promises. Biol. Psychiatry 88 , 818–828 (2020).
Gao, S., Greene, A. S., Constable, R. T. & Scheinost, D. Combining multiple connectomes improves predictive modeling of phenotypic measures. Neuroimage 201 , 116038 (2019).
Pujol, J. et al. Clinical application of functional magnetic resonance imaging in presurgical identification of the central sulcus. J. Neurosurg. 88 , 863–869 (1998).
Bullmore, E. The future of functional MRI in clinical medicine. Neuroimage 62 , 1267–1271 (2012).
Vanderwal, T., Kelly, C., Eilbott, J., Mayes, L. C. & Castellanos, F. X. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage 122 , 222–232 (2015).
Rosenberg, M., Noonan, S., DeGutis, J. & Esterman, M. Sustaining visual attention in the face of distraction: a novel gradual-onset continuous performance task. Atten. Percept. Psychophys. 75 , 426–439 (2013).
Pylyshyn, Z. W. & Storm, R. W. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spat. Vis. 3 , 179–197 (1988).
Luck, S. J. & Vogel, E. K. The capacity of visual working memory for features and conjunctions. Nature 390 , 279–284 (1997).
Pashler, H. Familiarity and visual change detection. Percept. Psychophys. 44 , 369–378 (1988).
Rouder, J. N., Morey, R. D., Morey, C. C. & Cowan, N. How to measure working memory capacity in the change detection paradigm. Psychon. Bull. Rev. 18 , 324–330 (2011).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29 , 162–173 (1996).
Shen, X., Tokoglu, F., Papademetris, X. & Constable, R. T. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage 82 , 403–415 (2013).
Scheinost, D. et al. Ten simple rules for predictive modeling of individual differences in neuroimaging. Neuroimage 193 , 35–45 (2019).
Nichols, T. E. & Holmes, A. P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15 , 1–25 (2002).
Abbas, K. et al. GEFF: graph embedding for functional fingerprinting. Neuroimage 221 , 117181 (2020).
Amico, E. & Goñi, J. The quest for identifiability in human functional connectomes. Sci. Rep. 8 , 8254 (2018).
Barch, D. M. et al. NeuroImage function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 80 , 169–189 (2013).
Smith, S. M. et al. Resting-state fMRI in the Human Connectome Project. Neuroimage 80 , 144–168 (2013).
Download references
Acknowledgements
This project was supported by National Institutes of Health grant MH108591 to M.M.C. and by National Science Foundation grant BCS1558497 to M.M.C.
Author information
Authors and affiliations.
Department of Psychology, Yale University, New Haven, CT, USA
Kwangsun Yoo, Monica D. Rosenberg, Young Hye Kwon, Qi Lin, Emily W. Avery & Marvin M. Chun
Department of Psychology, University of Chicago, Chicago, IL, USA
Monica D. Rosenberg
Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT, USA
Dustin Sheinost & R. Todd Constable
Interdepartmental Neuroscience Program, Yale University, New Haven, CT, USA
R. Todd Constable & Marvin M. Chun
Department of Neurosurgery, Yale School of Medicine, New Haven, CT, USA
R. Todd Constable
Department of Neuroscience, Yale School of Medicine, New Haven, CT, USA
Marvin M. Chun
Wu Tsai Institute, Yale University, New Haven, CT, USA
You can also search for this author in PubMed Google Scholar
Contributions
K.Y., M.D.R. and M.M.C. designed the study. Y.H.K. and E.W.A. performed fMRI experiments. K.Y. and M.D.R. analysed behavioural data. K.Y. and Y.H.K. analysed fMRI data. K.Y. conducted modelling and visualization. K.Y., M.M.C., M.D.R., Q.L., D.S. and R.T.C. discussed the results and implications. M.M.C. and R.T.C. supervised the project. K.Y., Y.H.K. and M.M.C. wrote the original draft; K.Y., M.M.C., M.D.R., Q.L., E.W.A., D.S. and R.T.C. reviewed the original draft and contributed to the final version of the paper.
Corresponding authors
Correspondence to Kwangsun Yoo or Marvin M. Chun .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Human Behaviour thanks Jing Sui, Francisco Castellanos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 predictive anatomy of three task-based cpms..
a . The scale bar in gradCPT, MOT and VSTM represents the relative ratio of predictive functional connections to all possible number of functional connections between networks with a sign representing whether the connection is in a positive or negative network. The scale bar in overlap represents the actual number of predictive functional connections with a sign representing whether the connection is in a positive or negative network. GradCPT: gradual-onset continuous performance task, MOT: multiple object tracking, and VSTM: visual short-term memory. MF: medial-frontal network, FP: frontoparietal network, DM: default mode network, VI: visual I, VII: visual II, VAs: visual association, SA: salience network, Subc: subcortex, Cbl: cerebellum. b . The number of predictive connections of three task-based CPMs in positive and negative networks.
Extended Data Fig. 2 Cross-prediction results of five common attention factor CPMs.
a . Cross-prediction results when models were applied to predict the common attention factor from different fMRI data. Models’ prediction accuracies were assessed by prediction q 2 and correlation r between observed and predicted common factor measures. P values of significance were obtained using 1,000 permutations and corrected for all 5×5 tests (***: p < 0.001, **: p < 0.01, *: p < 0.05, and ~: p < 0.1). Rows represent different fMRI data used to predict a common attention factor used in model construction, and columns represent the same but in model validation. b . Cross-prediction results, taking into account shared variance (the common factor) between task behaviors. Models’ prediction accuracies were assessed by partial correlation between observed and predicted behavior scores while controlling for the shared variance. P values of significance were obtained using 1,000 permutations and corrected for all 5×9 tests (***: p < 0.001, **: p < 0.01, *: p < 0.05, and ~: p < 0.1). Rows represent different fMRI data used to predict a common attention factor used in model construction, and columns represent combinations of fMRI data and behavior scores used in model validation. GradCPT: gradual-onset continuous performance task, MOT: multiple object tracking, and VSTM: visual short-term memory.
Extended Data Fig. 3 A similarity of individual behaviours between different tasks.
The similarity was assessed by Pearson’s correlation of individual performances between attention tasks. Individual behaviors were significantly correlated between every pair of tasks. GradCPT: gradual-onset continuous performance task, MOT: multiple object tracking, and VSTM: visual short-term memory.
Extended Data Fig. 4 Cross-prediction results of task-specific CPMs.
a . Cross-prediction results, taking into account shared variance between task behaviors. Models’ prediction accuracies were assessed by partial correlation between observed and predicted behavior scores while controlling for the shared variance. P value was obtained using 1,000 permutations and corrected for multiple tests (***: p < 0.001, **: p < 0.01, *: p < 0.05, and ~: p < 0.1). Rows represent combinations of fMRI data and behavior scores used in model construction, and columns represent combinations of fMRI data and behavior scores used in model validation. GradCPT: gradual-onset continuous performance task, MOT: multiple object tracking, and VSTM: visual short-term memory. b . Cross-prediction results when models were applied to predict the common attention factor from different fMRI data. Models’ prediction accuracies were assessed by correlation between observed and predicted common factor. P value was obtained using 1,000 permutations and corrected for all 9×5 tests (***: p < 0.001, **: p < 0.01, *: p < 0.05, and ~: p < 0.1). Rows represent combinations of fMRI data and behavior scores used in model construction, and columns represent different fMRI data used to predict a common attention factor used in model validation.
Extended Data Fig. 5 Cross-prediction using connectivity between the frontoparietal (FP, 2), visual II (VII, 6), salience (SA, 8), subcortical (Subc, 9), cerebellar (Cbl, 10) networks.
Prediction of a model using connectivity between the medial-frontal (1), default mode (3), motor (4), visual I (5), visual association (7) networks was also obtained as a control. A. Rows represent combinations of networks (indicated by numbers) used in each model. Models’ prediction accuracies were assessed by correlating model-predicted and observed behavioral scores. B. Prediction performance of each network obtained by averaging all models that used the network in A. C. The same result as A, but model accuracies were assessed by q2. D. Prediction performance of each network obtained by averaging all models that used the network in C. GradCPT: gradual-onset continuous performance task, MOT: multiple object tracking, and VSTM: visual short-term memory.
Extended Data Fig. 6 Similarity between C2C model-generated task connectomes and empirical task connectomes.
Error bar represents standard deviation from 1,000 iterations. A and C represent a spatial similarity between two connectomes assessed by Pearson’s correlation. Darker bars represent the similarity between empirical task and generated task connectomes, and lighter bars represent the similarity between empirical task and empirical rest connectomes. The higher similarity of the generated connectome indicates that the C2C model accurately generates the target task connectome from the rest connectome. B and D represent root mean square (RMS) difference between two connectomes. The smaller difference of the generated connectome indicates that the C2C model accurately generates the target task connectome from the rest connectome. In a box-whisker plot, a box covers the first to third quartile ( q 1 and q 3, respectively) of the data, and a center line represents the median. A red dot represents the mean. Whisker covers approximately 99.3% of data (±2.7* standrad deviation ), extended to the most extreme point that is not an outlier. A data point is considered an outlier if it is greater than q 3+1.5*( q 3- q 1) or less than q 1-1.5*( q 3- q 1). GradCPT: gradual-onset continuous performance task, MOT: multiple object tracking, and VSTM: visual short-term memory. *: p < 0.001 from 1,000 permutations.
Extended Data Fig. 7 The general attention connectome lookup table.
Out of a total 30,135 edges, 10,885 (36.1%) edges were pulled from gradCPT, 12,542 (41.6%) edges were from MOT, and 6,708 (22.3%) were from VSTM. The Ratio map was obtained based on All map. In each within- or between-network element in Ratio, the number of edges in the element for each task was counted and normalized by the total number of edges of each task. A task with the highest normalized value was assigned.
Extended Data Fig. 8 Scatter plots of predicted and observed attention scores in four external datasets.
Three models, the general attention model and two single task models (model 1 and 4 in Table 1) were trained within the internal dataset and then applied to rest connectomes in the four datasets. If a fitted line closely passes the origin (0,0) with a positive slope (staying within white quadrants), the model could be considered successfully predicting actual attentional abilities. There was no constraint on intercepts in fitting a line. The general model best generalized to predict various attentional measures in four independent external datasets.
Extended Data Fig. 9 Prediction error, assessed by mean square error (MSE), of the general attention model in four independent datasets.
The general model significantly reduced prediction error (assessed by MSE) compared to null models in four datasets. In all datasets, the general attention model produced the lowest prediction error among all models tested. ***: p < 0.001, **: p < 0.01, *: p < 0.05, and ~: p < 0.1 from 1,000 permutations.
Supplementary information
Supplementary methods, results, discussion, references, Tables 1–7 and Figs. 1–11.
Reporting summary
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Yoo, K., Rosenberg, M.D., Kwon, Y.H. et al. A brain-based general measure of attention. Nat Hum Behav 6 , 782–795 (2022). https://doi.org/10.1038/s41562-022-01301-1
Download citation
Received : 17 June 2021
Accepted : 14 January 2022
Published : 03 March 2022
Issue Date : June 2022
DOI : https://doi.org/10.1038/s41562-022-01301-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Integrating brain function and structure in the study of the human attentional networks: a functionnectome study.
- Mar Martín-Signes
- Pedro M. Paz-Alonso
- Ana B. Chica
Brain Structure and Function (2024)
Gaze-based attention refocusing training in virtual reality for adult attention-deficit/hyperactivity disorder
- Benjamin Selaskowski
- Laura Marie Asché
- Niclas Braun
BMC Psychiatry (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Browse Course Material
Course info.
- Prof. John D. E. Gabrieli
Departments
- Brain and Cognitive Sciences
As Taught In
- Cognitive Science
Learning Resource Types
Introduction to psychology.
« Previous | Next »
Session Overview
Session activities.
Read the following before watching the lecture video.
- Study outline for K&R Chapter 3 (PDF)
- [Stangor] Chapter 4, “Sensing and Perceiving”
Lecture Videos
View Full Video Lecture 7: Attention View by Chapter What Do We Mean by “Attention”? Demonstrations of the Limits of Attention Hypnosis and the Stroop Effect Visual Search and Attentional Blink Object Tracking and Improving Attention Subliminal Perception Video Resources Removed Clips Lecture Slides (PDF)
Discussion: Attention
Today we’re also going to talk about attention. How we engage with the world, but only a small part of it at a time. Why we can’t engage with the whole thing, and what it would be like to engage with the whole thing. Limits on our attention: why we can’t perceive everything at once…. Read more »
Check Yourself
Describe an example of selective attention. Does selective attention completely block out all other sensory input? If not give an example. What are the advantages and disadvantages of processes involved in selective attention?
› Sample Answer
One example of selective attention is the ability to focus on one person speaking in a crowd of people speaking. Selective attention does not completely block out all other sensory input. For example, the cocktail party phenomenon occurs when we are only attending to and aware of one person speaking. We seem to have no attention to the surrounding conversations. However, if someone says your name or something related to you, you quickly divert our attention to the person that said your name. Therefore, while consciously unaware of the surrounding sensory input, our brains are constantly monitory surrounding input.
The advantage of selective attention is that it allows us to focus on important elements of our environment while blocking out things that could be distracting. This is especially important for survival in dangerous situations. However, focusing too much on one thing could be a disadvantage if other things in the environment require more urgent focus. The “cocktail party phenomenon” is a process that allows us to monitor the environment while still focusing our attention on important things.
Further Study
These optional resources are provided for students that wish to explore this topic more fully.

You are leaving MIT OpenCourseWare

IMAGES
VIDEO
COMMENTS
The invisible gorilla experiment surprises everyone who hasn't heard about it before. Its results tell us about how our selective attention works.
List of top two psychological experiments on attention! Experiment # 1. Span of Attention – Visual: At any given moment there are several stimuli in the environment competing for our attention. However, our sense organs can respond to only a limited number of them at the same time. This limit is known as span of attention.
What is Selective Attention? Selective attention is the process of directing our awareness to relevant stimuli while ignoring irrelevant stimuli in the environment.
There is broad agreement that working memory is closely related to attention. This article delineates several theoretical options for conceptualizing this link, and evaluates their viability in light of their theoretical implications and the empirical support they received.
Within each of these, we address major classes of attention-demanding tasks which, in turn, spawn models and principles that can be used to mitigate attention problems when humans interact with technology outside the laboratory.
In 1999, Chris Chabris and Dan Simons conducted an experiment known as the “Invisible Gorilla Experiment.” They told participants they would watch a video of people passing around basketballs. In the middle of the video, a person in a gorilla suit walked through the circle momentarily.
It starts with an introduction to object-based attention and a description of the three commonly used experimental paradigms in object-based attention research. These are followed by a review of a variety of manifestations of object effects and the factors that influence object segmentation.
Attention has a ubiquitous role in perception and cognition 1. We are endlessly exposed to all kinds of overflowing sensory information, and the ability to deploy attention over space and to...
MIT OpenCourseWare is a web based publication of virtually all MIT course content. OCW is open and available to the world and is a permanent MIT activity.
E. Colin Cherry’s original 1953 selective attention experiment. The study describes an experiment involving dichotic listening, a demonstration of which was performed during lecture but removed for privacy reasons.