Skip to main content
- Agriculture + Food
- Arts + Society
- Business + Economy
- Engineering + Technology
- Environment + Sustainability
- Health + Medicine
- Resources + Energy
- Search news
- UQ responds

Mushrooms magnify memory by boosting nerve growth

Researchers from The University of Queensland have discovered the active compound from an edible mushroom that boosts nerve growth and enhances memory.
Professor Frederic Meunier from the Queensland Brain Institute said the team had identified new active compounds from the mushroom, Hericium erinaceus.
“Extracts from these so-called ‘lion’s mane’ mushrooms have been used in traditional medicine in Asian countries for centuries, but we wanted to scientifically determine their potential effect on brain cells,” Professor Meunier said.
“Pre-clinical testing found the lion’s mane mushroom had a significant impact on the growth of brain cells and improving memory.
“Laboratory tests measured the neurotrophic effects of compounds isolated from Hericium erinaceus on cultured brain cells, and surprisingly we found that the active compounds promote neuron projections, extending and connecting to other neurons.
“Using super-resolution microscopy, we found the mushroom extract and its active components largely increase the size of growth cones, which are particularly important for brain cells to sense their environment and establish new connections with other neurons in the brain.”
Co-author, UQ’s Dr Ramon Martinez-Marmol said the discovery had applications that could treat and protect against neurodegenerative cognitive disorders such as Alzheimer’s disease.
“Our idea was to identify bioactive compounds from natural sources that could reach the brain and regulate the growth of neurons, resulting in improved memory formation,” Dr Martinez-Marmol said.
Dr Dae Hee Lee from CNGBio Co, which has supported and collaborated on the research project, said the properties of lion’s mane mushrooms had been used to treat ailments and maintain health in traditional Chinese medicine since antiquity.
“This important research is unravelling the molecular mechanism of lion’s mane mushroom compounds and their effects on brain function, particularly memory,” Dr Lee said.
The study was published in the Journal of Neurochemistry .
UQ acknowledges the collaborative efforts of researchers from the Republic of Korea’s Gachon University and Chungbuk National University.
Media: QBI Communications, [email protected] u , Elaine Pye +61 415 222 606, Merrett Pye +61 422 096 049.
- Share on Twitter
- Share on Facebook
- Google Plus
Health + Medicine , Research
Experts , Health + Medicine , Research

Health + Medicine , Research , Resources + Energy

Research , Awards and Achievements

Arts + Society , Health + Medicine , Research

Health + Medicine , Research , Research Grants , Uni news
Recent headlines.

- UQ’s commitment to inclusion recognised 7 June 2024
The Conversation
Peter dutton’s latest salvo on australia’s emissions suggests our climate wars are far from over, weakening or collapse of a major atlantic current has disrupted nz’s climate in the past – and could do so again, michael mosley used science communication to advance health and wellbeing. we can learn a lot from his approach, how a culturally informed model of care helped first nations patients with heart disease, what will a robot make of your résumé the bias problem with using ai in job recruitment.
+61 7 3365 1111
Other Campuses: UQ Gatton , UQ Herston
Maps and Directions
© 2024 The University of Queensland
A Member of
Privacy & Terms of use | Feedback
Authorised by: Director, Office of Marketing and Communications
ABN : 63 942 912 684 CRICOS Provider No: 00025B
Quick Links
- Emergency Contact
Social Media
- Giving to UQ
- Faculties & Divisions
- UQ Contacts
Ph. 3365 3333

Mushrooms Magnify Memory by Boosting Nerve Growth
Summary: Active compounds in the edible Lion’s Mane mushroom can help promote neurogenesis and enhance memory, a new study reports. Preclinical trials report the compound had a significant impact on neural growth and improved memory formation. Researchers say the compound could have clinical applications in treating and preventing neurodegenerative disorders such as Alzheimer’s disease.
Source: University of Queensland
Researchers from The University of Queensland have discovered the active compound from an edible mushroom that boosts nerve growth and enhances memory.
Professor Frederic Meunier from the Queensland Brain Institute said the team had identified new active compounds from the mushroom, Hericium erinaceus.
“Extracts from these so-called ‘lion’s mane’ mushrooms have been used in traditional medicine in Asian countries for centuries, but we wanted to scientifically determine their potential effect on brain cells,” Professor Meunier said. “Pre-clinical testing found the lion’s mane mushroom had a significant impact on the growth of brain cells and improving memory. “Laboratory tests measured the neurotrophic effects of compounds isolated from Hericium erinaceus on cultured brain cells, and surprisingly we found that the active compounds promote neuron projections, extending and connecting to other neurons. “Using super-resolution microscopy, we found the mushroom extract and its active components largely increase the size of growth cones, which are particularly important for brain cells to sense their environment and establish new connections with other neurons in the brain.”

Co-author, UQ’s Dr Ramon Martinez-Marmol said the discovery had applications that could treat and protect against neurodegenerative cognitive disorders such as Alzheimer’s disease. “Our idea was to identify bioactive compounds from natural sources that could reach the brain and regulate the growth of neurons, resulting in improved memory formation,” Dr Martinez-Marmol said. Dr Dae Hee Lee from CNGBio Co, which has supported and collaborated on the research project, said the properties of lion’s mane mushrooms had been used to treat ailments and maintain health in traditional Chinese medicine since antiquity. “This important research is unravelling the molecular mechanism of lion’s mane mushroom compounds and their effects on brain function, particularly memory,” Dr Lee said. The study was published in the Journal of Neurochemistry . UQ acknowledges the collaborative efforts of researchers from the Republic of Korea’s Gachon University and Chungbuk National University.
About this neurogenesis and memory research news
Author: Elaine Pye Source: University of Queensland Contact: Elaine Pye – University of Queensland Image: The image is in the public domain
Original Research: Open access. “ Hericerin derivatives activates a pan-neurotrophic pathway in central hippocampal neurons converging to ERK1/2 signaling enhancing spatial memory ” by Frederic Meunier et al. Journal of Neurochemistry
Hericerin derivatives activates a pan-neurotrophic pathway in central hippocampal neurons converging to ERK1/2 signaling enhancing spatial memory
The traditional medicinal mushroom Hericium erinaceus is known for enhancing peripheral nerve regeneration through targeting nerve growth factor (NGF) neurotrophic activity.
Here, we purified and identified biologically new active compounds from H. erinaceus , based on their ability to promote neurite outgrowth in hippocampal neurons. N -de phenylethyl isohericerin (NDPIH), an isoindoline compound from this mushroom, together with its hydrophobic derivative hericene A, were highly potent in promoting extensive axon outgrowth and neurite branching in cultured hippocampal neurons even in the absence of serum, demonstrating potent neurotrophic activity.
Pharmacological inhibition of tropomyosin receptor kinase B (TrkB) by ANA-12 only partly prevented the NDPIH-induced neurotrophic activity, suggesting a potential link with BDNF signaling. However, we found that NDPIH activated ERK1/2 signaling in the absence of TrkB in HEK-293T cells, an effect that was not sensitive to ANA-12 in the presence of TrkB.
Our results demonstrate that NDPIH acts via a complementary neurotrophic pathway independent of TrkB with converging downstream ERK1/2 activation. Mice fed with H. erinaceus crude extract and hericene A also exhibited increased neurotrophin expression and downstream signaling, resulting in significantly enhanced hippocampal memory.
Hericene A therefore acts through a novel pan-neurotrophic signaling pathway , leading to improved cognitive performance.
Is it better to eat lion mane cooked or pill form I love mushrooms
It’ll be very good too, for TBI and aphasia cuz I have that now.
I wonder if using the term neurogenesis in this context is misleading. This supplement would seem to stimulate connectivity between existing neurons; not to create new ones. Be that as it may, enhanced connectivity can be beneficial. The danger, of course, is increased stimulation can negatively impact established pathways and over-stimulation can actually disrupt them to the point of neurosis.
A study sponsored by a company that sells the product being tested is bad enough, but the study is admittedly “sub-clinical” – there is no testing nor proof mentioned that the compounds/chemicals producing these study results would have any effect on human brains following digestion, either as fresh produce or as a powder or pills. By extension, there’s no indication how much of these chemicals would have to be consumed to have a similar positive effect, and what the possible negative effects of that level of consumption might be. And do the same chemicals also promote accelerated growth of tumor cells? The headline here and in multiple sites picking up this story is misleading at best, but will generate revenue for the sponsoring company as well as the ad revenue for the clickbait. If there is a website that lists these studies and has a yes/no column indicating financial interest/sponsorship, I’d appreciate someone posting the link.
There are other studies that say the same things. Perhaps you could read some before telling people it’s bunk when you clearly don’t know anything at all about the muschrooms.
I have tried dozens of supplements to help me continue to play bullet chess online (each side has 1 minute total for all their moves), even though I am too old for it (54). Lion’s Mane is one of my keepers. I have been taking it for about 2 years. I was never stronger before the last 2 years. And I have been very active playing bullet chess for around 16 years (well over 200,000 games). Lion’s Mane Mushroom extract, Salmon oil, Nicotinamide Riboside, PQQ, Lecithin, B-2, L-Carnosine, Choline Bitartrate, and DMAE appear to be effective for me. And raspberry Ice Tea or Hibiscus Tea.
Thanks, Noodle -Naut! Any particular brand of Lion’s Mane extract?
from where do you get these supplements?
RealMushrooms.com is the best source
So Noodle … are you going to share a Brand Name and where we might find it available?
That’s a lot of information with no payoff so far.
Please construct this eb page so that I can forward it to my wife ‘ just love mushrooms!
Comments are closed.
Brain Misleads Sight: New Insights on Visual Illusions

How the Internet Warps Human Morality

Virtual Rat with AI Brain Mimics Real Rodent Movement

1 in 4 People with Bipolar Disorder Achieve Complete Mental Health
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
The acute and chronic effects of lion’s mane mushroom supplementation on cognitive function, stress and mood in young adults: a double-blind, parallel groups, pilot study.

1. Introduction
2. materials and methods, 2.1. study design and participants, 2.2. treatments, 2.3. cognitive and mood assessments, 2.4. procedure, 2.5. statistics, 3.1. the acute effects of lion’s mane after a single dose (day 1), 3.2. the chronic effects of lion’s mane 28-day treatment, 4. discussion, 5. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Ainsworth, G.C. Ainsworth & Bisby’s Dictionary of the Fungi ; Cabi: Wallingford, UK, 2008. [ Google Scholar ]
- Index, F. Hericium erinaceus (Bull.) Pers., Comm. Fung. Clav. (Lipsiae): 27 (1797). Available online: https://www.indexfungorum.org/names/NamesRecord.asp?RecordID=356812 (accessed on 2 November 2023).
- Ghosh, S.; Nandi, S.; Banerjee, A.; Sarkar, S.; Chakraborty, N.; Acharya, K. Prospecting medicinal properties of Lion’s mane mushroom. J. Food Biochem. 2021 , 45 , e13833. [ Google Scholar ] [ CrossRef ]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus , an amazing medicinal mushroom. Mycol. Prog. 2015 , 14 , 1–23. [ Google Scholar ] [ CrossRef ]
- Li, W.; Zhou, W.; Kim, E.-J.; Shim, S.H.; Kang, H.K.; Kim, Y.H. Isolation and identification of aromatic compounds in Lion’s Mane Mushroom and their anticancer activities. Food Chem. 2015 , 170 , 336–342. [ Google Scholar ] [ CrossRef ]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015 , 63 , 7108–7123. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus : An edible mushroom with medicinal values. J. Complement. Integr. Med. 2013 , 10 , 253–258. [ Google Scholar ] [ CrossRef ]
- Cheng, J.-H.; Tsai, C.-L.; Lien, Y.-Y.; Lee, M.-S.; Sheu, S.-C. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement. Altern. Med. 2016 , 16 , 1–9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Spelman, K.; Sutherland, E.; Bagade, A. Neurological activity of Lion’s mane ( Hericium erinaceus ). J. Restor. Med. 2017 , 6 , 19–26. [ Google Scholar ] [ CrossRef ]
- Hu, J.-H.; Li, I.-C.; Lin, T.-W.; Chen, W.-P.; Lee, L.-Y.; Chen, C.-C.; Kuo, C.-F. Absolute bioavailability, tissue distribution, and excretion of erinacine S in Hericium erinaceus mycelia. Molecules 2019 , 24 , 1624. [ Google Scholar ] [ CrossRef ]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum . Tetrahedron Lett. 1994 , 35 , 1569–1572. [ Google Scholar ] [ CrossRef ]
- Zhang, C.-C.; Yin, X.; Cao, C.-Y.; Wei, J.; Zhang, Q.; Gao, J.-M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorganic Med. Chem. Lett. 2015 , 25 , 5078–5082. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.; Bua, O.; Di Mauro, P.; Toscano, M.; Petralia, C.; Maiolino, L. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016 , 13 , 1–11. [ Google Scholar ] [ CrossRef ]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.-J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016 , 23 , 1–12. [ Google Scholar ] [ CrossRef ]
- Wang, L.-Y.; Huang, C.-S.; Chen, Y.-H.; Chen, C.-C.; Chen, C.-C.; Chuang, C.-H. Anti-inflammatory effect of erinacine C on NO production through down-regulation of NF-κB and activation of Nrf2-mediated HO-1 in BV2 microglial cells treated with LPS. Molecules 2019 , 24 , 3317. [ Google Scholar ] [ CrossRef ]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of Hericium erinaceus on amyloid β (25-35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011 , 32 , 67–72. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tsai, Y.-C.; Lin, Y.-C.; Huang, C.-C.; Villaflores, O.B.; Wu, T.-Y.; Huang, S.-M.; Chin, T.-Y. Hericium erinaceus mycelium and its isolated compound, erinacine A, ameliorate high-fat high-sucrose diet-induced metabolic dysfunction and spatial learning deficits in aging mice. J. Med. Food 2019 , 22 , 469–478. [ Google Scholar ] [ CrossRef ]
- Martínez-Mármol, R.; Chai, Y.; Khan, Z.; Kim, S.B.; Hong, S.M.; Gormal, R.S.; Lee, D.H.; Lee, J.K.; Lee, M.K.; Kim, S.Y. Hericerin derivatives from Hericium erinaceus exert bdnf-like neurotrophic activity in central hippocampal neurons and enhance memory. bioRxiv 2020 , 2008 , 271676. [ Google Scholar ]
- Vigna, L.; Morelli, F.; Agnelli, G.M.; Napolitano, F.; Ratto, D.; Occhinegro, A.; Di Iorio, C.; Savino, E.; Girometta, C.; Brandalise, F. Hericium erinaceus improves mood and sleep disorders in patients affected by overweight or obesity: Could circulating pro-BDNF and BDNF be potential biomarkers? Evid. Based Complement. Altern. Med. 2019 , 2019 , 7861297. [ Google Scholar ] [ CrossRef ]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake ( Hericium erinaceus ) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009 , 23 , 367–372. [ Google Scholar ] [ CrossRef ]
- Li, I.; Chang, H.-H.; Lin, C.-H.; Chen, W.-P.; Lu, T.-H.; Lee, L.-Y.; Chen, Y.-W.; Chen, Y.-P.; Chen, C.-C.; Lin, D.P.-C. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020 , 12 , 155. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of cognitive functions by oral intake of Hericium erinaceus . Biomed. Res. 2019 , 40 , 125–131. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grozier, C.D.; Alves, V.A.; Killen, L.G.; Simpson, J.D.; O’Neal, E.K.; Waldman, H.S. Four Weeks of Hericium erinaceus Supplementation Does Not Impact Markers of Metabolic Flexibility or Cognition. Int. J. Exerc. Sci. 2022 , 15 , 1366. [ Google Scholar ] [ PubMed ]
- Huang, C.-J.; Webb, H.E.; Zourdos, M.C.; Acevedo, E.O. Cardiovascular reactivity, stress, and physical activity. Front. Physiol. 2013 , 4 , 314. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Webb, H.E.; Weldy, M.L.; Fabianke-Kadue, E.C.; Orndorff, G.; Kamimori, G.H.; Acevedo, E.O. Psychological stress during exercise: Cardiorespiratory and hormonal responses. Eur. J. Appl. Physiol. 2008 , 104 , 973–981. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010 , 31 , 231–237. [ Google Scholar ] [ CrossRef ]
- Browne, R.H. On the use of a pilot sample for sample size determination. Stat. Med. 1995 , 14 , 1933–1940. [ Google Scholar ] [ CrossRef ]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. J. Appl. Stat. Pharm. Ind. 2005 , 4 , 287–291. [ Google Scholar ] [ CrossRef ]
- Jackson, P.A.; Wightman, E.L.; Veasey, R.; Forster, J.; Khan, J.; Saunders, C.; Mitchell, S.; Haskell-Ramsay, C.F.; Kennedy, D.O. A randomized, crossover study of the acute cognitive and cerebral blood flow effects of phenolic, nitrate and botanical beverages in young, healthy humans. Nutrients 2020 , 12 , 2254. [ Google Scholar ] [ CrossRef ]
- Kennedy, D.O.; Jackson, P.A.; Forster, J.; Khan, J.; Grothe, T.; Perrinjaquet-Moccetti, T.; Haskell-Ramsay, C.F. Acute effects of a wild green-oat ( Avena sativa ) extract on cognitive function in middle-aged adults: A double-blind, placebo-controlled, within-subjects trial. Nutr. Neurosci. 2017 , 20 , 135–151. [ Google Scholar ] [ CrossRef ]
- Kennedy, D.O.; Wightman, E.L.; Forster, J.; Khan, J.; Haskell-Ramsay, C.F.; Jackson, P.A. Cognitive and mood effects of a nutrient enriched breakfast bar in healthy adults: A randomised, double-blind, placebo-controlled, parallel groups study. Nutrients 2017 , 9 , 1332. [ Google Scholar ] [ CrossRef ]
- Wightman, E.L.; Jackson, P.A.; Spittlehouse, B.; Heffernan, T.; Guillemet, D.; Kennedy, D.O. The Acute and Chronic Cognitive Effects of a Sage Extract: A Randomized, Placebo Controlled Study in Healthy Humans. Nutrients 2021 , 13 , 218. [ Google Scholar ] [ CrossRef ]
- Wightman, E.L.; Jackson, P.A.; Forster, J.; Khan, J.; Wiebe, J.C.; Gericke, N.; Kennedy, D.O. Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2020 , 12 , 2194. [ Google Scholar ] [ CrossRef ]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983 , 24 , 385–396. [ Google Scholar ] [ CrossRef ]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019 , 13 , 363. [ Google Scholar ] [ CrossRef ]
- Brenner, E.K.; Weigand, A.J.; Edwards, L.; Thomas, K.R.; Edmonds, E.C.; Bondi, M.W.; Bangen, K.J. Brain Derived Neurotrophic Factor Interacts with White Matter Hyperintensities to Influence Processing Speed and Hippocampal Volume in Older Adults. J. Alzheimers Dis. 2023 , 93 , 141–149. [ Google Scholar ] [ CrossRef ]
- Chong, P.S.; Fung, M.-L.; Wong, K.H.; Lim, L.W. Therapeutic potential of Hericium erinaceus for depressive disorder. Int. J. Mol. Sci. 2020 , 21 , 163. [ Google Scholar ] [ CrossRef ]
- Ma, B.-J.; Shen, J.-W.; Yu, H.-Y.; Ruan, Y.; Wu, T.-T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus . Mycology 2010 , 1 , 92–98. [ Google Scholar ] [ CrossRef ]
- Kawagishi, H.; Shimada, A.; Hosokawa, S.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum . Tetrahedron Lett. 1996 , 37 , 7399–7402. [ Google Scholar ] [ CrossRef ]
- Ryu, S.; Kim, H.G.; Kim, J.Y.; Kim, S.Y.; Cho, K.-O. Hericium erinaceus extract reduces anxiety and depressive behaviors by promoting hippocampal neurogenesis in the adult mouse brain. J. Med. Food 2018 , 21 , 174–180. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wiener, C.D.; de Mello Ferreira, S.; Moreira, F.P.; Bittencourt, G.; de Oliveira, J.F.; Molina, M.L.; Jansen, K.; de Mattos Souza, L.D.; Lara, D.R.; Portela, L.V. Serum levels of nerve growth factor (NGF) in patients with major depression disorder and suicide risk. J. Affect. Disord. 2015 , 184 , 245–248. [ Google Scholar ] [ CrossRef ]
- Diniz, B.S.; Teixeira, A.L.; Machado-Vieira, R.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Reduced serum nerve growth factor in patients with late-life depression. Am. J. Geriatr. Psychiatry 2013 , 21 , 493–496. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chiu, C.-H.; Chyau, C.-C.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Liu, J.-L.; Lin, W.-H.; Mong, M.-C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3β signaling in mice. Int. J. Mol. Sci. 2018 , 19 , 341. [ Google Scholar ] [ CrossRef ]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020 , 25 , 2251–2274. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jackson, P.A.; Forster, J.; Khan, J.; Pouchieu, C.; Dubreuil, S.; Gaudout, D.; Moras, B.; Pourtau, L.; Joffre, F.; Vaysse, C. Effects of saffron extract supplementation on mood, well-being, and response to a psychosocial stressor in healthy adults: A randomized, double-blind, parallel group, clinical trial. Front. Nutr. 2021 , 7 , 365. [ Google Scholar ] [ CrossRef ]
- Kennedy, D.O.; Bonnländer, B.; Lang, S.C.; Pischel, I.; Forster, J.; Khan, J.; Jackson, P.A.; Wightman, E.L. Acute and chronic effects of green oat ( Avena sativa ) extract on cognitive function and mood during a laboratory stressor in healthy adults: A randomised, double-blind, placebo-controlled study in healthy humans. Nutrients 2020 , 12 , 1598. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dodd, F.; Kennedy, D.; Wightman, E.; Khan, J.; Patan, M.; Elcoate, R.; Jackson, P. The chronic effects of a combination of herbal extracts (Euphytose ® ) on psychological mood state and response to a laboratory stressor: A randomised, placebo-controlled, double blind study in healthy humans. J. Psychopharmacol. 2022 , 36 , 1243–1256. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rönnlund, M.; Nyberg, L.; Bäckman, L.; Nilsson, L.-G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychol. Aging 2005 , 20 , 3. [ Google Scholar ] [ CrossRef ]
- Grassi, D.; Socci, V.; Tempesta, D.; Ferri, C.; De Gennaro, L.; Desideri, G.; Ferrara, M. Flavanol-rich chocolate acutely improves arterial function and working memory performance counteracting the effects of sleep deprivation in healthy individuals. J. Hypertens. 2016 , 34 , 1298–1308. [ Google Scholar ] [ CrossRef ]
- Jackson, P.A.; Haskell-Ramsay, C.; Forster, J.; Khan, J.; Veasey, R.; Kennedy, D.O.; Wilson, A.R.; Saunders, C.; Wightman, E.L. Acute cognitive performance and mood effects of coffee berry and apple extracts: A randomised, double blind, placebo controlled crossover study in healthy humans. Nutr. Neurosci. 2022 , 25 , 2335–2343. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2010 , 24 , 1505–1514. [ Google Scholar ] [ CrossRef ]
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Docherty, S.; Doughty, F.L.; Smith, E.F. The Acute and Chronic Effects of Lion’s Mane Mushroom Supplementation on Cognitive Function, Stress and Mood in Young Adults: A Double-Blind, Parallel Groups, Pilot Study. Nutrients 2023 , 15 , 4842. https://doi.org/10.3390/nu15224842
Docherty S, Doughty FL, Smith EF. The Acute and Chronic Effects of Lion’s Mane Mushroom Supplementation on Cognitive Function, Stress and Mood in Young Adults: A Double-Blind, Parallel Groups, Pilot Study. Nutrients . 2023; 15(22):4842. https://doi.org/10.3390/nu15224842
Docherty, Sarah, Faye L. Doughty, and Ellen F. Smith. 2023. "The Acute and Chronic Effects of Lion’s Mane Mushroom Supplementation on Cognitive Function, Stress and Mood in Young Adults: A Double-Blind, Parallel Groups, Pilot Study" Nutrients 15, no. 22: 4842. https://doi.org/10.3390/nu15224842
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 161 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Open access
- Published: 07 June 2024
Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: a randomized, controlled clinical trial
- Dean Ornish 1 , 2 ,
- Catherine Madison 1 , 3 ,
- Miia Kivipelto 4 , 5 , 6 , 7 ,
- Colleen Kemp 8 ,
- Charles E. McCulloch 9 ,
- Douglas Galasko 10 ,
- Jon Artz 11 , 12 ,
- Dorene Rentz 13 , 14 , 15 ,
- Jue Lin 16 ,
- Kim Norman 17 ,
- Anne Ornish 1 ,
- Sarah Tranter 8 ,
- Nancy DeLamarter 1 ,
- Noel Wingers 1 ,
- Carra Richling 1 ,
- Rima Kaddurah-Daouk 18 ,
- Rob Knight 19 ,
- Daniel McDonald 20 ,
- Lucas Patel 21 ,
- Eric Verdin 22 , 23 ,
- Rudolph E. Tanzi 13 , 24 , 25 , 26 &
- Steven E. Arnold 13 , 27
Alzheimer's Research & Therapy volume 16 , Article number: 122 ( 2024 ) Cite this article
14k Accesses
726 Altmetric
Metrics details
Evidence links lifestyle factors with Alzheimer’s disease (AD). We report the first randomized, controlled clinical trial to determine if intensive lifestyle changes may beneficially affect the progression of mild cognitive impairment (MCI) or early dementia due to AD.
A 1:1 multicenter randomized controlled phase 2 trial, ages 45-90 with MCI or early dementia due to AD and a Montreal Cognitive Assessment (MoCA) score of 18 or higher. The primary outcome measures were changes in cognition and function tests: Clinical Global Impression of Change (CGIC), Alzheimer’s Disease Assessment Scale (ADAS-Cog), Clinical Dementia Rating–Sum of Boxes (CDR-SB), and Clinical Dementia Rating Global (CDR-G) after 20 weeks of an intensive multidomain lifestyle intervention compared to a wait-list usual care control group. ADAS-Cog, CDR-SB, and CDR-Global scales were compared using a Mann-Whitney-Wilcoxon rank-sum test, and CGIC was compared using Fisher’s exact test. Secondary outcomes included plasma Aβ42/40 ratio, other biomarkers, and correlating lifestyle with the degree of change in these measures.
Fifty-one AD patients enrolled, mean age 73.5. No significant differences in any measures at baseline. Only two patients withdrew. All patients had plasma Aβ42/40 ratios <0.0672 at baseline, strongly supporting AD diagnosis. After 20 weeks, significant between-group differences in the CGIC ( p = 0.001), CDR-SB ( p = 0.032), and CDR Global ( p = 0.037) tests and borderline significance in the ADAS-Cog test ( p = 0.053). CGIC, CDR Global, and ADAS-Cog showed improvement in cognition and function and CDR-SB showed significantly less progression, compared to the control group which worsened in all four measures. Aβ42/40 ratio increased in the intervention group and decreased in the control group ( p = 0.003). There was a significant correlation between lifestyle and both cognitive function and the plasma Aβ42/40 ratio. The microbiome improved only in the intervention group ( p <0.0001).
Conclusions
Comprehensive lifestyle changes may significantly improve cognition and function after 20 weeks in many patients with MCI or early dementia due to AD.
Trial registration
Approved by Western Institutional Review Board on 12/31/2017 (#20172897) and by Institutional Review Boards of all sites. This study was registered retrospectively with clinicaltrials.gov on October 8, 2020 (NCT04606420, ID: 20172897).
Increasing evidence links lifestyle factors with the onset and progression of dementia, including AD. These include unhealthful diets, being sedentary, emotional stress, and social isolation.
For example, a Lancet commission on dementia prevention, intervention, and care listed 12 potentially modifiable risk factors that together account for an estimated 40% of the global burden of dementia [ 1 ]. Many of these factors (e.g., hypertension, smoking, depression, type 2 diabetes, obesity, physical inactivity, and social isolation) are also risk factors for coronary heart disease and other chronic illnesses because they share many of the same underlying biological mechanisms. These include chronic inflammation, oxidative stress, insulin resistance, telomere shortening, sympathetic nervous system hyperactivity, and others [ 2 ]. A recent study reported that the association of lifestyle with cognition is mostly independent of brain pathology, though a part, estimated to be only 12%, was through β-amyloid [ 3 ].
In one large prospective study of adults 65 or older in Chicago, the risk of developing AD was 38% lower in those eating high vs low amounts of vegetables and 60% lower in those consuming omega-3 fatty acids at least once/week, [ 4 ] whereas consuming saturated fat and trans fats more than doubled the risk of developing AD [ 5 ].A systematic review and meta-analysis of 243 observational prospective studies and 153 randomized controlled trials found a similar relationship between these and similar risk factors and the onset of AD [ 6 ].
The multifactorial etiology and heterogeneity of AD suggest that multidomain lifestyle interventions may be more effective than single-domain ones for reducing the risk of dementia, and that more intensive multimodal lifestyle interventions may be more efficacious than moderate ones at preventing dementia [ 7 ].
For example, in the Finnish Geriatric Intervention Study (FINGER) study, a RCT of men and women 60-77 in age with Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE) dementia risk scores of at least 6 points and cognition at mean or slightly lower, a multimodal intervention of diet, exercise, cognitive training, vascular risk monitoring maintained cognitive function after 2 years in older adults at increased risk of dementia [ 8 ]. After 24 months, global cognition in the FINGER intervention group was 25% higher than in the control group which declined. Moreover, the FINGER intervention was equally beneficial regardless of several demographic and socioeconomic risk factors [ 9 ] and apolipoprotein E (APOE) ε4 status [ 10 ].
The FINGER lifestyle intervention also resulted in a 13-20% reduction in rates of cardiovascular disease events (stroke, transient ischemic attack, or coronary), providing more evidence that “what’s good for the heart is good for the brain”(and vice versa) [ 11 ]. Other large-scale multidomain intervention studies to determine if this intervention can help prevent dementia are being conducted or planned in over 60 countries worldwide, as part of the World-Wide FINGERS network, including the POINTER study in the U.S. [ 12 , 13 ].
More recently, a similar dementia prevention-oriented RCT showed that a 2-year personalized multidomain intervention led to modest improvements in cognition and dementia risk factors in those at risk for (but not diagnosed with) dementia and AD [ 14 ].
All these studies showed that lifestyle changes may help prevent dementia. The study we are reporting here is the first randomized, controlled clinical trial to test whether intensive lifestyle changes may beneficially affect those already diagnosed with mild cognitive impairment (MCI) or early dementia due to AD.
In two earlier RCTs, we found that the same multimodal lifestyle intervention described in this article resulted in regression of coronary atherosclerosis as measured by quantitative coronary arteriography [ 15 ] and ventricular function, [ 16 ] improvements in myocardial perfusion as measured by cardiac PET scans, and 2.5 times fewer cardiac events after five years, all of which were statistically significant [ 17 ]. Until then, it was believed that coronary heart disease progression could only be slowed, not stopped or reversed, similar to how MCI or early dementia due to AD are viewed today.
Since AD and coronary heart disease share many of the same risk factors and biological mechanisms, and since moderate multimodal lifestyle changes may help prevent AD, [ 18 ] we hypothesized that a more intensive multimodal intervention proven to often reverse the progression of coronary heart disease and some other chronic diseases may also beneficially affect the progression of MCI or early dementia due to AD.
We report here results of a randomized controlled trial to determine if the progression of MCI or early dementia due to AD may be slowed, stopped, or perhaps even reversed by a comprehensive, multimodal, intensive lifestyle intervention after 20 weeks when compared to a usual-care randomized control group. This lifestyle intervention includes (1) a whole foods, minimally processed plant-based diet low in harmful fats and low in refined carbohydrates and sweeteners with selected supplements; (2) moderate exercise; (3) stress management techniques; and (4) support groups.
This intensive multimodal lifestyle modification RCT sought to address the following questions:
Can the specified multimodal intensive lifestyle changes beneficially affect the progression of MCI or early dementia due to AD as measured by the AD Assessment Scale–Cognitive Subscale (ADAS-Cog), CGIC (Clinical Global Impression of Change), CDR-SB (Clinical Dementia Rating Sum of Boxes), and CDR-G (Clinical Dementia Rating Global) testing?
Is there a significant correlation between the degree of lifestyle change and the degree of change in these measures of cognition and function?
Is there a significant correlation between the degree of lifestyle change and the degree of change in selected biomarkers (e.g., the plasma Aβ42/40 ratio)?
Participants and methods
This study was a 1:1 multi-center RCT during the first 20 weeks of the study, and these findings are reported here. Patients who met the clinical trial inclusion criteria were enrolled between September 2018 and June 2022.
Participants were enrolled who met the following inclusion criteria:
Male or female, ages 45 to 90
Current diagnosis of MCI or early dementia due to AD process, with a MoCA score of 18 or higher (National Institute on Aging–Alzheimer’s Association McKhann and Albert 2011 criteria) [ 19 , 20 ]
Physician shared this diagnosis with the patient and approved their participation in this clinical trial
Willingness and ability to participate in all aspects of the intervention
Availability of spouse or caregiver to provide collateral information and assist with study adherence
Patients were excluded if they had any of the following:
Moderate or severe dementia
Physical disability that precludes regular exercise
Evidence for other primary causes of neurodegeneration or dementia, e.g., significant cerebrovascular disease (whose primary cause of dementia was vascular in origin), Lewy Body disease, Parkinson's disease, FTD
Significant ongoing psychiatric or substance abuse problems
Fifty-one participants with MCI or early-stage dementia due to AD who met these inclusion criteria were enrolled between September 2018 and June 2022 and underwent baseline testing. 26 of the enrolled participants were randomly assigned to an intervention group that received the multimodal lifestyle intervention for 20 weeks and 25 participants were randomly assigned to a usual habits and care control group that was asked not to make any lifestyle changes for 20 weeks, after which they would be offered the intervention. Patients in both groups received standard of care treatment managed by their own neurologist.
The intervention group received the lifestyle program for 20 weeks (initially in person, then via synchronous Zoom after March 2020 due to COVID-19). Two participants who did not want to continue these lifestyle changes withdrew during this time, both in the intervention group (one male, one female). Participants in both groups completed a follow-up visit at 20 weeks, where clinical and cognitive assessments were completed. Data were analyzed comparing the baseline and 20 week assessments between the groups.
In a drug trial, access to an investigational new drug can be restricted from participants in a randomized control group. However, we learned in our prior clinical trials of this lifestyle intervention with other diseases that it is often difficult to persuade participants who are randomly assigned to a usual-care control group to refrain from making these lifestyle changes for more than 20 weeks, which is why this time duration was chosen. If participants in both groups made similar lifestyle changes, then it would not be possible to show differences between the groups. Therefore, to encourage participants randomly assigned to the control group not to make lifestyle changes during the first 20 weeks, we offered to provide them the same lifestyle program at no cost to them for 20 weeks after being in the usual-care control group and tested after 20 weeks.
We initially planned to enroll 100 patients into this study based on power calculations of possible differences between groups in cognition and function after 20 weeks. However, due to challenges in recruiting patients, especially with the COVID-19 emergency and that many pharma trials began recruiting patients with similar criteria, it took longer to enroll patients than initially planned [ 21 ]. Because of this, we terminated recruitment after 51 patients were enrolled. This decision was based only on recruitment issues and limited funding, without reviewing the data at that time.
Patients were recruited from advertisements, presentations at neurology meetings, referrals from diverse groups of neurologists and other physicians, and a search of an online database of patients at UCSF. We put a special emphasis on recruiting diverse patients, although we were less successful in doing so than we hoped (Table 1 ).
This clinical trial was approved by the Western Institutional Review Board on 12/31/2017 (approval number: 20172897) and all participants and their study partners provided written informed consent. The trial protocol was also approved by the appropriate Institutional Review Board of all participating sites, and all subjects provided informed consent. Due to the COVID-19 emergency, planned MRI and amyloid PET scans were no longer feasible, and the number of cognition and function tests was decreased. An initial inclusion criterion of “current diagnosis of mild to moderate dementia due to AD (McKhann et al., 2011)” was further clarified to include a MoCA score of 18 or higher. This study was registered with clinicaltrials.gov on October 8, 2020 (NCT04606420, Unique Protocol ID: 20172897) retrospectively due to an administrative error. None of the sponsors who provided funding for this study participated in its design, conduct, management, or reporting of the results. Those providing the lifestyle intervention were separate from those performing testing and from those collecting and analyzing the data, who were blinded to group assignment. All authors contributed to manuscript draft revisions, provided critical comment, and approved submission for publication.
Any modifications in the protocol were approved in advance and in writing by the senior biostatistician (Charles McCulloch PhD) or the senior expert neuropsychologist (Dorene Rentz PsyD), and subsequently approved by the WIRB.
Patients were initially recruited only from the San Francisco Bay area beginning October 2018 and met in person until February 2020 when the COVID-19 pandemic began. Subsequently, this multimodal lifestyle intervention was offered to patients at home in real time via Zoom.
Offering this intervention virtually provided an opportunity to recruit patients from multiple sites, including the Massachusetts General Hospital/Harvard Medical School, Boston, MA; the University of California, San Diego; and Renown Regional Medical Center, Reno, NV, as well as with neurologists in the San Francisco Bay Area. These participants were recruited and tested locally at each site and the intervention was provided via Zoom and foods were sent directly to their home.
Patient recruitment
This is described in the Supplemental Materials section.
Intensive multimodal lifestyle intervention
Each patient received a copy of a book which describes this lifestyle medicine intervention for other chronic diseases. [ 2 ]
A whole foods minimally-processed plant-based (vegan) diet, high in complex carbohydrates (predominantly fruits, vegetables, whole grains, legumes, soy products, seeds and nuts) and especially low in harmful fats, sweeteners and refined carbohydrates. It was approximately 14-18% of calories as total fat, 16-18% protein, and 63-68% mostly complex carbohydrates. Calories were unrestricted. Those with higher caloric needs were given extra portions.
To assure the high adherence and standardization required to adequately test the hypothesis, 21 meals/week and snacks plus the daily supplements listed below were provided throughout the 40 weeks of this intervention to each study participant and his or her spouse or study partner at no cost to them. Twice/week, we overnight shipped to each patient as well as to their spouse or study partner three meals plus two snacks per day that met the nutritional guidelines as well as the prescribed nutritional supplements.
We asked participants to consume only the food and nutritional supplements we sent to them and no other foods. We reasoned that if adherence to the diet and lifestyle intervention was high, whatever outcomes we measured would be of interest. That is, if patients in the intervention group were adherent but showed no significant benefits, that would be a disappointing but an important finding. If they showed improvement, that would also be an important finding. But if they did not follow the lifestyle intervention sufficiently, then we would not have been able to adequately test the hypotheses.
Aerobic (e.g., walking) at least 30 minutes/day and mild strength training exercises at least three times per week from an exercise physiologist in person or with virtual sessions. Patients were given a personalized exercise prescription based on age and fitness level. All sessions were overseen by a registered nurse.
- Stress management
Meditation, gentle yoga-based poses, stretching, progressive relaxation, breathing exercises, and imagery for a total of one hour per day, supervised by a certified stress management specialist. The purpose of each technique was to increase the patient’s sense of relaxation, concentration, and awareness. They were also given access to online meditations. Patients had the option of using flashing-light glasses at a theta frequency of 7.83 Hz plus soothing music as an aid to meditation and insomnia [ 22 ]. They were also encouraged to get adequate sleep.
Group support
Participants and their spouses/study partners participated in a support group one hour/session, three days/week, supervised by a licensed mental health professional in a supportive, safe environment to increase emotional support and community as well as communication skills and strategies for maintaining adherence to the program. They also received a book with memory exercises used periodically during group sessions [ 23 ].
To reinforce this lifestyle intervention, each patient and their spouse or study partner met three times/week, four hours/session via Zoom: 2
one hour of supervised exercise (aerobic + strength training)
one hour of stress management practices (stretching, breathing, meditation, imagery)
one hour of a support group
one hour lecture on lifestyle
Additional optional exercise and stress management classes were provided.
Supplements
Omega-3 fatty acids with Curcumin (1680 mg omega-3 & 800 mg Curcumin, Nordic Naturals ProOmega CRP, 4 capsules/day). Omega-3 fatty acids: In those age 65 or older, those consuming omega-3 fatty acids once/week or more had a 60% lower risk of developing AD, and total intake of n-3 polyunsaturated fatty acids was associated with reduced risk of Alzheimer disease [ 24 ]. Curcumin targets inflammatory and antioxidant pathways as well as (directly) amyloid aggregation, [ 25 ] although there may be problems with bioavailability and crossing the blood-brain barrier [ 26 ].
Multivitamin and Minerals (Solgar VM-75 without iron, 1 tablet/day). Combinatorial formulations demonstrate improvement in cognitive performance and the behavioral difficulties that accompany AD [ 27 ].
Coenzyme Q10 (200 mg, Nordic Naturals, 2 soft gels/day). CoQ10. May reduce mitochondrial impairment in AD [ 28 ].
Vitamin C (1 gram, Solgar, 1 tablet/day): Maintaining healthy vitamin C levels may have a protective function against age-related cognitive decline and AD [ 29 ].
Vitamin B12 (500 mcg, Solgar, 1 tablet/day): B12 hypovitaminosis is linked to the development of AD pathology [ 30 ].
Magnesium L-Threonate (Mg) (144 mg, Magtein, 2 tablets/day). A meta-analysis found that Mg deficiency may be a risk factor of AD and Mg supplementation may be an adjunctive treatment for AD [ 31 ].
Hericium erinaceus (Lion’s Mane, Stamets Host Defense, 2 grams/day): Lion’s mane may produce significant improvements in cognition and function in healthy people over 50 [ 32 ] and in MCI patients compared to placebo [ 33 ].
Super Bifido Plus Probiotic (Flora, 1 tablet/day). A meta-analysis suggests that probiotics may benefit AD patients [ 34 ].
Primary outcome measures: cognition and function testing
Four tests were used to assess changes in cognition and function in these patients. These are standard measures of cognition and function included in many FDA drug trials: ADAS-Cog; Clinical Global Impression of Change (CGIC); Clinical Dementia Rating Sum of Boxes (CDR-SB); Clinical Dementia Rating Global (CDR Global). All cognition and function raters were trained psychometrists with experience in administering these tests in clinical trials. Efforts were made to have the same person perform cognitive testing at each visit to reduce inter-observer variability. Those doing ADAS-Cog assessments were certified raters and tested patients in person. The CGIC and CDR tests were administered for all patients via Zoom by different raters than the ADAS-cog. Also, raters were blind to treatment arm to the degree possible.
Secondary outcome measures: biomarkers and microbiome
These are described in the Supplemental Materials section. These include blood-based biomarkers (such as the plasma Aβ42/40 ratio) and microbiome taxa (organisms).
Statistical methods
These are described in the Supplemental Materials section.
The recruitment effort for this trial lasted from 01/23/2018 to 6/16/2022. The most effective recruitment method was referral from the subjects’ physician or healthcare provider. Additional recruitment efforts included advertising in print and digital media; speaking to community groups; mentioning the study during podcast and radio interviews; collaborating with research institutions that provide dementia diagnosis and treatment; and contracting a clinical trials recruitment service (Linea). A total of 1585 people contacted us; of these, 1300 did not meet the inclusion criteria, 102 declined participation, and 132 were screening incomplete when enrollment closed, resulting in the enrollment of 51 participants (Fig. 1 ).
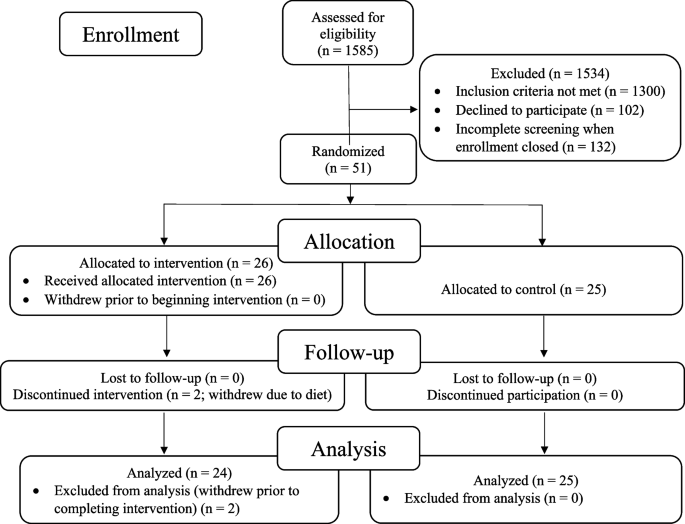
CONSORT flowchart: patients, demographics, and enrollment
The remaining 51 patients were randomized to an intervention group (26 patients) that received the lifestyle intervention for 20 weeks or to a usual-care control group (25 patients) that was asked not to make any lifestyle changes. Two patients in the intervention group withdrew during the intervention because they did not want to continue the diet and lifestyle changes. No patients in the control group withdrew prior to 20-week testing. Analyses were performed on the remaining 49 patients. No patients were lost to follow-up.
All of these 49 patients had plasma Aβ42/40 ratios <0.089 (all were <0.0672), strongly supporting the diagnosis of Alzheimer’s disease [ 35 ].
At baseline, there were no statistically significant differences between the intervention group and the randomized control group in any measures, including demographic characteristics, cognitive function measures, or biomarkers (Table 1 and Table 2 ).
Cognition and function testing: primary analysis
Results after 20 weeks of a multimodal intensive lifestyle intervention in all patients showed overall statistically significant differences between the intervention group and the randomized control group in cognition and function in the CGIC ( p = 0.001), CDR-SB ( p = 0.032), and CDR Global ( p = 0.037) tests and of borderline significance in the ADAS-Cog test ( p = 0.053, Table 3 ). Three of these measures (CGIC, CDR Global, ADAS-Cog) showed improvement in cognition and function in the intervention group and worsening in the control group, and one test (CDR-SB) showed significantly less progression when compared to the randomized control group, which worsened in all four of these measures.
PRIMARY ANALYSIS (with outlier included), Table 3 :
CGIC (Clinical Global Impression of Change)
These scores improved in the intervention group and worsened in the control group.
(Fisher’s exact p -value = 0.001). 10 people in the intervention group showed improvement compared to none in the control group. 7 people in the intervention group and 8 people in the control group were unchanged. 7 people in the intervention group showed minimal worsening compared to 14 in the control group. None in the intervention group showed moderate worsening compared to 3 in the control group.
CDR-Global (Clinical Dementia Rating-Global)
These scores improved in the intervention group (from 0.69 to 0.65) and worsened in the randomized control group (from 0.66 to 0.74), mean difference = 0.12, p = 0.037 (Table 3 and Fig. 2 ).
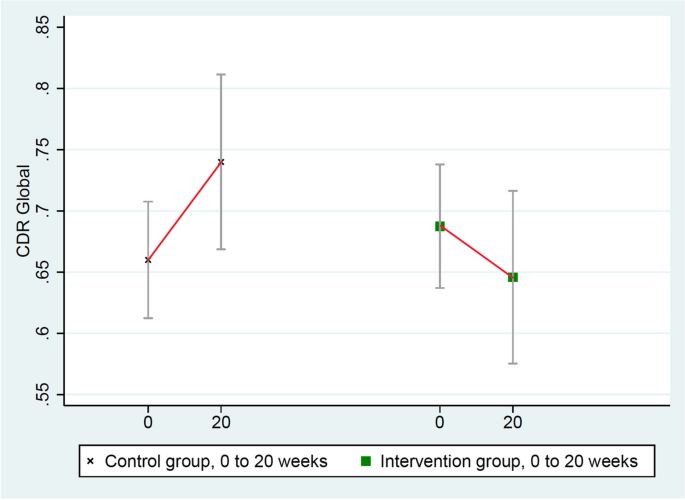
Changes in CDR-Global (lower = improved)
ADAS-Cog (Alzheimer’s Disease Assessment Scale)
These scores improved in the intervention group (from 21.551 to 20.536) and worsened in the randomized control group (from 21.252 to 22.160), mean group difference of change = 1.923 points, p = 0.053 (Table 3 and Fig. 3 ). (ADAS-Cog testing in one intervention group patient was not administered properly so it was excluded.)
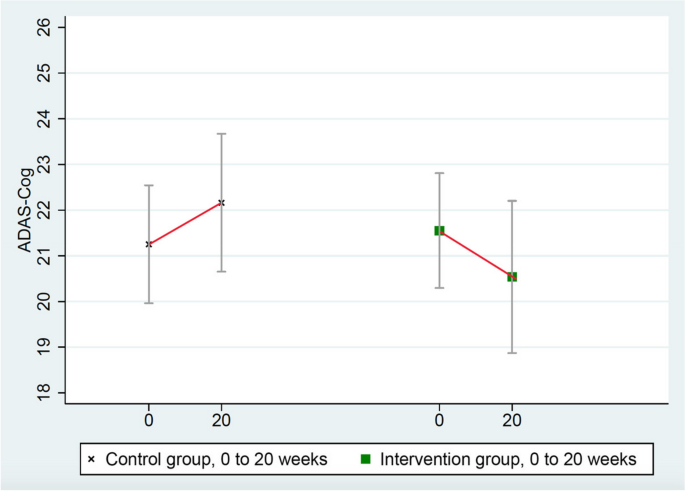
Changes in ADAS-Cog (lower = improved)
CDR-SB (Clinical Dementia Rating Sum of Boxes)
These scores worsened significantly more in the control group (from 3.34 to 3.86) than in the intervention group (from 3.27 to 3.35), mean group difference = 0.44, p = 0.032 (Table 3 and Fig. 4 ).
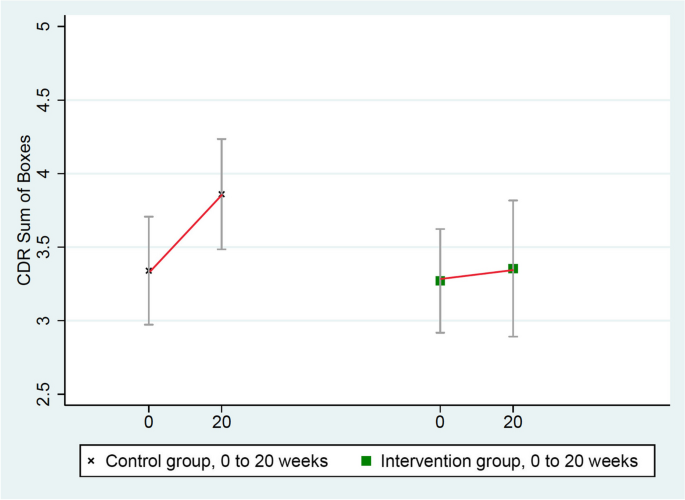
Changes in CDR-SB (lower = improved)
There were no significant differences in depression scores as measured by PHQ-9 between the intervention and control groups.
Secondary sensitivity analyses
One patient in the intervention group was a clear statistical outlier in his cognitive function testing based on standard mathematical definitions (none was an outlier in the control group) [ 36 ]. Therefore, this patient’s data were excluded in a secondary sensitivity analysis. These results showed statistically significant differences in all four of these measures of cognition and function (Table 4 ). Three measures (ADAS-Cog, CGIC, and CDR Global) showed significant improvement in cognition and function and one (CDR-SB) showed significantly less worsening when compared to the randomized control group, which worsened in all four of these measures.
Sensitivity analysis (with outlier excluded)
There were no significant differences in depression scores as measured by PHQ-9 between the intervention and control groups in either analysis.
A reason why this patient might have been a statistical outlier is that he reported intense situational stress before his testing. As a second sensitivity analysis, this same outlier patient was retested when he was calmer, and all four measures (ADAS-Cog, CGIC, CDR Global, and CDR-SB) showed significant improvement in cognition and function, whereas the randomized control group worsened in all four of these measures.
Biomarker results
We selected biomarkers that have a known role in the pathophysiology of AD (Table 5 ). Of note is that the plasma Aβ42/40 ratio increased in the intervention group but decreased in the randomized control group ( p = 0.003, two-tailed).
Correlation of lifestyle index and cognitive function
In the current clinical trial, despite the inherent limitations of self-reported data, we found statistically significant correlations between the degree of lifestyle change (from baseline to 20 weeks) and the degree of change in three of four measures of cognition and function as well as correlations between the adherence to desired lifestyle changes at just the 20-week timepoint and the degree of change in two of the four measures of cognition and function and borderline significance in the fourth measure.
Correlation with lifestyle at 20 weeks: p = 0.052; correlation: 0.241
Correlation with degree of change in lifestyle: p = 0.015; correlation: 0.317
Correlation with lifestyle at 20 weeks: p = 0.043; correlation: 0.251
Correlation with degree of change in lifestyle: p = 0.081; correlation: 0.205
Correlation with lifestyle at 20 weeks: p = 0.065; correlation: 0.221
Correlation with degree of change in lifestyle: p = 0.024; correlation: 0.286
Correlation with lifestyle at 20 weeks: p = 0.002
Correlation with degree of change in lifestyle: p = 0.0005
(CGIC tests are non-parametric analyses, so standard effect size calculations are not included for this measure.)
Also, we also found a significant correlation between dietary total fat intake and changes in the CGIC measure ( p = 0.001), but this was not significant for the other three measures.
Correlation of lifestyle index and biomarker data
In the current clinical trial, despite the inherent limitations of self-reported data, we found statistically significant correlations between the degree of lifestyle change (from baseline to 20 weeks) and the degree of change in many of the key biomarkers, as well as correlations between the degree of lifestyle change at 20 weeks and the degree of change in these biomarkers:
Plasma Aβ42/40 ratio
Correlation with lifestyle at 20 weeks: p = 0.035; correlation: 0.306
Correlation with degree of change in lifestyle: p = 0.068; correlation: 0.266
Correlation with lifestyle at 20 weeks: p = 0.011; correlation: 0.363
Correlation with degree of change in lifestyle: p = 0.007; correlation: 0.383
LDL-cholesterol
Correlation with lifestyle at 20 weeks: p < 0.0001; correlation: 0.678
Correlation with degree of change in lifestyle: p < 0.0001; correlation: 0.628
Beta-Hydroxybutyrate (ketones)
Correlation with lifestyle at 20 weeks: p = 0.013; correlation: 0.372
Correlation with degree of change in lifestyle: p = 0.034; correlation: 0.320
Correlation with lifestyle at 20 weeks: p = 0.228; correlation: 0.177
Correlation with degree of change in lifestyle: p = 0.135; correlation: 0.219
GFAP/glial fibrillary acidic protein
Correlation with lifestyle at 20 weeks: p = 0.096; correlation: 0.243
Correlation with degree of change in lifestyle: p =0.351; correlation: 0.138
What degree of lifestyle change is correlated with improvement in cognitive function tests?
What degree of lifestyle is needed to stop or improve the worsening of MCI or early dementia due to AD? In other words, what % of adherence to the lifestyle intervention was correlated with no change in MCI or dementia across both groups? Higher adherence than this degree of lifestyle change was associated with improvement in MCI or dementia.
Correlation with lifestyle at 20 weeks: 71.4% adherence
Correlation with lifestyle at 20 weeks: 120.6% adherence
CDR-Global:
Correlation with lifestyle at 20 weeks: 95.6%
Microbiome results
There was a significant and beneficial change in the microbiome configuration in the intervention group but not in the control group.
Several taxa (groups of microorganisms) that increased only in the intervention group were consistent with those involved in reduced AD risk in other studies. For example, Blautia, which increased during the intervention in the intervention group, has previously been associated with a lower risk of AD, potentially due to its involvement in increasing γ-aminobutyric acid (GABA) production [ 37 ]. Eubacterium also increased during the intervention in the intervention group, and prior studies have identified Eubacterium genera (namely Eubacterium fissicatena) as a protective factor in AD [ 38 ].
Also, there was a decrease in relative abundance of taxa involved in increased AD risk in the intervention group, e.g., Prevotella and Turicibacter , the latter of which has been associated with relevant biological processes such as 5-HT production. Prevotella and Turicibacter have previously been shown to increase with disease progression, [ 39 ] and these taxa decreased over the course of the intervention.
These results support the hypothesis that the lifestyle intervention may beneficially modify specific microbial groups in the microbiome: increasing those that lower the risk of AD and decreasing those that increase the risk of AD. (Please see Supplement for more detailed information.)
We report the first randomized, controlled trial showing that an intensive multimodal lifestyle intervention may significantly improve cognition and function and may allay biological features in many patients with MCI or early dementia due to AD after 20 weeks.
After 20 weeks of a multimodal intensive lifestyle intervention, results of the primary analysis when all patients were included showed overall statistically significant differences between the intervention group and the randomized control group in cognition and function as measured by the CGIC ( p = 0.001), CDR-SB ( p = 0.032), and CDR Global ( p = 0.037) tests and of borderline significance in the ADAS-Cog test ( p = 0.053).
Three of these measures (CGIC, CDR Global, ADAS-Cog) showed improvement in cognition and function in the intervention group and worsening in the randomized control group, and one test (CDR-SB) showed less progression in the intervention group when compared to the control group which worsened in all four of these measures.
These differences were even clearer in a secondary sensitivity analysis when a mathematical outlier was excluded. These results showed statistically significant differences between groups in all four of these measures of cognition and function. Three of these measures showed improvement in cognition and function and one (CDR-SB) showed less deterioration when compared to the randomized control group, which worsened in all four of these measures.
The validity of these changes in cognition and function and possible biological mechanisms of improvement is supported by the observed changes in several clinically relevant biomarkers that showed statistically significant differences in a beneficial direction after 20 weeks when compared to the randomized control group.
One of the most clinically relevant biomarkers is the plasma Aβ42/40 ratio, which increased by 6.4% in the intervention group and decreased by 8.3% in the randomized control group after 20 weeks, and these differences were statistically significant ( p = 0.003, two-tailed).
In the lecanemab trial, plasma levels of the Aβ42/40 biomarker increased in the intervention group over 18 months with the presumption that this reflected amyloid moving from the brain to the plasma [ 40 ]. We found similar results in the direction of change in the plasma Aβ42/40 ratio from this lifestyle intervention but in only 20 weeks. Conversely, this biomarker decreased in the control group (as in the lecanemab trial), which may indicate increased cerebral uptake of amyloid.
Other clinically relevant biomarkers also showed statistically significant differences (two-tailed) in a beneficial direction after 20 weeks when compared to the randomized control group. These include hemoglobin A1c, insulin, glycoprotein acetyls (GlycA), LDL-cholesterol, and β-Hydroxybutyrate (ketone bodies).
Improvement in these biomarkers provides more biological plausibility for the observed improvements in cognition and function as well as more insight into the possible mechanisms of improvement. This information may also help in predicting which patients are more likely to show improvements in cognition and function by making these intensive lifestyle changes.
Other relevant biomarkers were in a beneficial direction of change in the intervention group compared with the randomized control group after 20 weeks. These include pTau181, GFAP, CRP, SAA, and C-peptide. Telomere length increased in the intervention group and was essentially unchanged in the control group. These differences were not statistically significant even when there was an order of magnitude difference between groups (as with GFAP and pTau181) or an almost four-fold difference (as with CRP), but these changes were in a beneficial direction. At least in part, these findings may be due to a relatively small sample size and/or a short duration of only 20 weeks.
We found a statistically significant dose-response correlation between the degree of lifestyle changes in both groups (“lifestyle index”) and the degree of change in many of these biomarkers. This correlation was found in both the degree of change in lifestyle from baseline to 20 weeks as well as the lifestyle measured at 20 weeks. These correlations also add to the biological plausibility of these findings.
We also found a statistically significant dose-response correlation between the degree of lifestyle changes in both groups (“lifestyle index”) and changes in most measures of cognition and function testing. In short, the more these AD patients changed their lifestyle in the prescribed ways, the greater was the beneficial impact on their cognition and function. These correlations also add to the biological plausibility of these findings. This variation in adherence helps to explain in part why some patients in the intervention group improved and others did not, but there are likely other mechanisms that we do not fully understand that may play a role. These statistically significant correlations are especially meaningful given the greater variability of self-reported data, the relatively small sample size, and the short duration of the intervention.
These findings are consistent with earlier clinical trials in which we used this same lifestyle intervention and the same measure of lifestyle index and found significant dose-response correlations between this lifestyle index (i.e., the degree of lifestyle changes) and changes in the degree of coronary atherosclerosis (percent diameter stenosis) in coronary heart disease; [ 41 , 45 ] changes in PSA levels and LNCaP cell growth in men with prostate cancer; [ 42 ] and changes in telomere length [ 43 ].
We also found significant differences between the intervention and control groups in several taxa (groups of micro-organisms) in the microbiome which may be beneficial.
There were no significant differences in depression scores as measured by PHQ-9 between the intervention and control groups. Therefore, reduction in depression is unlikely to account for the overall improvements in cognition and function seen in the intervention group patients.
We also found that substantial lifestyle changes were required to stop the progression of MCI in these patients. In the primary analysis, this ranged from 71.4% adherence for ADAS-Cog to 95.6% adherence for CDR-Global to 120.6% adherence for CDR-SB. In other words, extensive lifestyle changes were required to stop or improve cognition and function in these patients. This helps to explain why other studies of less-intensive lifestyle interventions may not have been sufficient to stop deterioration or improve cognition and function.
For example, comparing these results to those of the MIND-AD clinical trial provides more biological plausibility for both studies [ 44 ]. That is, more moderate multimodal lifestyle changes may slow the rate of worsening of cognition and function in MCI or early dementia due to early-stage AD, whereas more intensive multimodal lifestyle changes may result in overall average improvements in many measures of cognition and function when compared to a randomized usual-care control group in both clinical trials.
Lifestyle changes may provide additional benefits to patients on drug therapy. Anti-amyloid antibodies have shown modest effects on slowing progression, but they are expensive, have potential for adverse events, are not yet widely available, and do not result in overall cognitive improvement [ 40 ]. Perhaps there may be synergy from doing both.
Limitations
This study has several limitations. Only 51 patients were enrolled and randomized in our study, and two of these patients (both in the intervention group) withdrew during the trial. Showing statistically significant differences across different tests of cognition and function and other measures despite the relatively small sample size suggests that the lifestyle intervention may be especially effective and has strong internal validity.
However, the smaller sample size limits generalizability, especially since there was much less racial and ethnic diversity in this sample than we strived to achieve. Also, we measured these differences despite the relative insensitivity of these measures, which might have increased the likelihood of a type II error.
Raters were blinded to the group assignment of the participants. However, unlike a double-blind placebo-controlled drug trial, it is not possible to blind subjects in a lifestyle intervention about whether or not they are receiving the intervention. This might have affected outcome measures, although to reduce positive expectations and because it was true, patients were told during the study that we did not know whether or not this lifestyle intervention would be beneficial, and we said that whatever we showed would be useful.
Also, 20 weeks is a relatively short time for any intervention with MCI or early dementia due to AD. We did not include direct measures of brain structure in this trial, so we cannot determine whether there were direct impacts on markers of brain pathology relevant to AD. However, surrogate markers such as the plasma Aβ42/40 ratio are becoming more widely accepted.
Not all patients in the intervention group improved. Of the 24 patients in the intervention group, 10 showed improvement as measured by the CGIC test, 7 were unchanged, and 7 worsened. In the control group, none improved, 8 were unchanged, and 17 worsened. In part, this may be explained by variations in adherence to the lifestyle intervention, as there was a significant relationship between the degree of lifestyle change and the degree of change in cognition and function across both groups. We hope that further research may further clarify other factors and mechanisms to help explain why cognition and function improved in some patients but not in others.
The findings on the degree of lifestyle change required to stop the worsening or improve cognition and function need to be interpreted with caution. Since data from both groups were combined, it was no longer a randomized trial for this specific analysis, so there could be unknown confounding influences. Also, it is possible that those with improved changes in cognition were better able to adhere to the intervention and thus have higher lifestyle indices.
In summary, in persons with mild cognitive impairment or early dementia due to Alzheimer’s disease, comprehensive lifestyle changes may improve cognition and function in several standard measures after 20 weeks. In contrast, patients in the randomized control group showed overall worsening in all four measures of cognition and function during this time.
The validity of these findings was supported by the observed changes in plasma biomarkers and microbiome; the dose-response correlation of the degree of lifestyle change with the degree of improvement in all four measures of cognition and function; and the correlation between the degree of lifestyle change and the degree of changes in the Aβ42/40 ratio and the changes in some other relevant biomarkers in a beneficial direction.
Our findings also have implications for helping to prevent AD. Newer technologies, some aided by artificial intelligence, enable the probable diagnosis of AD years before it becomes clinically apparent. However, many people do not want to know if they are likely to get AD if they do not believe they can do anything about it. If intensive lifestyle changes may cause improvement in cognition and function in MCI or early dementia due to AD, then it is reasonable to think that these lifestyle changes may also help to prevent MCI or early dementia due to AD. Also, it may take less-extensive lifestyle changes to help prevent AD than to treat it. Other studies cited earlier on the effects of these lifestyle changes on diseases such as coronary heart disease support this conclusion. Clearly, intensive lifestyle changes rather than moderate ones seem to be required to improve cognition and function in those suffering from early-stage AD.
These findings support longer follow-up and larger clinical trials to determine the longer-term outcomes of this intensive lifestyle medicine intervention in larger groups of more diverse AD populations; why some patients beneficially respond to a lifestyle intervention better than others besides differences in adherence; as well as the potential synergy of these lifestyle changes and some drug therapies.
Availability of data and materials
The datasets used and/or analyzed during the current study may be available from the corresponding author on reasonable request. Requesters will be asked to submit a study protocol, including the research question, planned analysis, and data required. The authors will evaluate this plan (i.e., relevance of the research question, suitability of the data, quality of the proposed analysis, planned or ongoing analysis, and other matters) on a case-by-case basis.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/S0140-6736(20)30367-6 . (Epub 2020 Jul 30. Erratum in: Lancet. 2023 Sep 30;402(10408):1132. PMID: 327389 PMCID: PMC7392084).
Article PubMed PubMed Central Google Scholar
Ornish D, Ornish A. UnDo It. New York: Ballantine Books; 2019.
Google Scholar
Dhana K, Agarwal P, James BD, Leurgans SE, Rajan KB, Aggarwal NT, Barnes LL, Bennett DA, Schneider JA. Healthy Lifestyle and Cognition in Older Adults With Common Neuropathologies of Dementia. JAMA Neurol. 2024. https://doi.org/10.1001/jamaneurol.2023.5491 . Epub ahead of print. PMID: 38315471.
Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370–6. https://doi.org/10.1212/01.wnl.0000240224.38978.d8 . (PMID:17060562;PMCID:PMC3393520).
Article CAS PubMed Google Scholar
Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60(2):194–200. https://doi.org/10.1001/archneur.60.2.194 . (Erratum.In:ArchNeurol.2003Aug;60(8):1072 PMID: 12580703).
Article PubMed Google Scholar
Yu JT, Xu W, Tan CC, Andrieu S, Suckling J, Evangelou E, Pan A, Zhang C, Jia J, Feng L, Kua EH, Wang YJ, Wang HF, Tan MS, Li JQ, Hou XH, Wan Y, Tan L, Mok V, Tan L, Dong Q, Touchon J, Gauthier S, Aisen PS, Vellas B. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91(11):1201–9. https://doi.org/10.1136/jnnp-2019-321913 . (Epub 2020 Jul 20. PMID: 32690803; PMCID: PMC7569385).
Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Lin PH, Liao L, et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology. 2019;92(3):e212–23. https://doi.org/10.1212/WNL.0000000000006784 . (Epub 2018/12/21. PubMed PMID: 30568005; PubMed Central PMCID: PMCPMC6340382).
Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63. https://doi.org/10.1016/S0140-6736(15)60461-5 . (Epub 2015 Mar 12 PMID: 25771249).
Rosenberg A, Ngandu T, Rusanen M, Antikainen R, Backman L, Havulinna S, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers Dement. 2018;14(3):263–70. https://doi.org/10.1016/j.jalz.2017.09.006 . (Epub 2017/10/23. PubMed PMID: 29055814).
Solomon A, Turunen H, Ngandu T, Peltonen M, Levalahti E, Helisalmi S, et al. Effect of the apolipoprotein e genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75(4):462–70. https://doi.org/10.1001/jamaneurol.2017.4365 . (Epub 2018/01/23. PubMed PMID: 29356827; PubMed Central PMCID: PMCPMC5885273).
Lehtisalo J, Rusanen M, Solomon A, Antikainen R, Laatikainen T, Peltonen M, et al. Effect of a multi-domain lifestyle intervention on cardiovascular risk in older people: the FINGER trial. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehab922 . Epub 2022/01/21. PubMed PMID: 35051281.
Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078–94. https://doi.org/10.1002/alz.12123 . (Epub 2020/07/07. PubMed PMID: 32627328).
Kivipelto M, Mangialasche F, Snyder H M, Allegri R, Andrieu S, Arai H, Baker L, Belleville S, Brodaty H, Brucki SM, Calandri I, Caramelli P, Chen C, Chertkow H, Chew E, Choi S H, Chowdhary N, Crivelli L, De La Torre R, Du Y, Dua T, Espeland M, Feldman H H, Hartmanis M, Hartmann T, Heffernan M, Henry C J, Hong C H, Håkansson K, Iwatsubo T, Jeong J H, Jimenez‐Maggiora G, Koo E H, Launer L J, Lehtisalo J, Lopera F, Martínez‐Lage P, Martins R, Middleton L, Molinuevo J L, Montero‐Odasso M, Moon S Y, Morales‐Pérez K, Nitrini R, Nygaard H B, Park Y K, Peltonen M, Qiu C, Quiroz Y T, Raman R, Rao N, Ravindranath V, Rosenberg A, Sakurai T, Salinas R M, Scheltens P, Sevlever G, Soininen H, Sosa A L, Suemoto C K, Tainta‐Cuezva M, Velilla L, Wang Y, Whitmer R, Xu X, Bain L J, Solomon A, Ngandu T, Carillo, M C. World‐Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimer's Dement. 2020, https://doi.org/10.1002/alz.12123 .
Yaffe K, Vittinghoff E, Dublin S, Peltz CB, Fleckenstein LE, Rosenberg DE, Barnes DE, Balderson BH, Larson EB. Effect of personalized risk-reduction strategies on cognition and dementia risk profile among older adults: the SMARRT randomized clinical trial. JAMA Intern Med. 2023:e236279. https://doi.org/10.1001/jamainternmed.2023.6279 . Epub ahead of print. PMID: 38010725; PMCID: PMC10682943
Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–7. https://doi.org/10.1001/jama.280.23.2001 . (Erratum.In:JAMA1999Apr21;281(15):1380 PMID: 9863851).
Ornish D, Scherwitz LW, Doody RS, Kesten D, McLanahan SM, Brown SE, DePuey E, Sonnemaker R, Haynes C, Lester J, McAllister GK, Hall RJ, Burdine JA, Gotto AM Jr. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54–9 (PMID: 6336794).
Gould KL, Ornish D, Scherwitz L, Brown S, Edens RP, Hess MJ, Mullani N, Bolomey L, Dobbs F, Armstrong WT, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA. 1995;274(11):894–901. https://doi.org/10.1001/jama.1995.03530110056036 . (PMID: 7674504).
Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology. 2020;95(4):e374–83. https://doi.org/10.1212/WNL.0000000000009816 . (Epub 2020 Jun 17. PMID: 32554763; PMCID: PMC7455318).
Article CAS PubMed PubMed Central Google Scholar
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. https://doi.org/10.1016/j.jalz.2011.03.005 . (Epub 2011 Apr 21. PMID: 21514250; PMCID: PMC3312024).
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
McDonald K, Seltzer E, Lu M, Gaisenband SD, Fletcher C, McLeroth P, Saini KS. Quantifying the impact of the COVID-19 pandemic on clinical trial screening rates over time in 37 countries. Trials. 2023;24(1):254. https://doi.org/10.1186/s13063-023-07277-1 . (PMID:37013558;PMCID:PMC10071259).
Tang HY, Vitiello MV, Perlis M, Mao JJ, Riegel B. A pilot study of audio-visual stimulation as a self-care treatment for insomnia in adults with insomnia and chronic pain. Appl Psychophysiol Biofeedback. 2014;39(3–4):219–25. https://doi.org/10.1007/s10484-014-9263-8 . (PMID:25257144;PMCID:PMC4221414).
Horsley K. Unlimited Memory. Granger Indiana: TCK Publishing; 2016.
Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60(7):940–6. https://doi.org/10.1001/archneur.60.7.940 . (Epub 2003/07/23. PubMed PMID: 12873849).
Voulgaropoulou SD, van Amelsvoort T, Prickaerts J, Vingerhoets C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019;1725:146476. https://doi.org/10.1016/j.brainres.2019.146476 . Epub 2019/09/29. PubMedPMID:31560864.
Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, Gylys KH, Badmaev V, Heath DD, Apostolova LG, Porter V, Vanek Z, Marshall GA, Hellemann G, Sugar C, Masterman DL, Montine TJ, Cummings JL, Cole GM. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012;4(5):43. https://doi.org/10.1186/alzrt146 . (PMID:23107780;PMCID:PMC3580400).
Shea TB, Remington R. Nutritional supplementation for Alzheimer’s disease? Curr Opin Psychiatry. 2015;28(2):141–7. https://doi.org/10.1097/YCO.0000000000000138 . (Epub 2015/01/21. PubMed PMID: 25602242).
Pradhan N, Singh C, Singh A. Coenzyme Q10 a mitochondrial restorer for various brain disorders. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(11):2197–222. https://doi.org/10.1007/s00210-021-02161-8 . (Epub 2021/10/02 PubMed PMID: 34596729).
Harrison FE. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J Alzheimers Dis. 2012;29(4):711–26. https://doi.org/10.3233/JAD-2012-111853 . (Epub 2012/03/01. PubMed PMID: 22366772; PubMed Central PMCID: PMCPMC3727637).
Lauer AA, Grimm HS, Apel B, Golobrodska N, Kruse L, Ratanski E, et al. Mechanistic Link between Vitamin B12 and Alzheimer's Disease. Biomolecules. 2022;12(1). https://doi.org/10.3390/biom12010129 . Epub 2022/01/22. PubMed PMID: 35053277; PubMed Central PMCID: PMCPMC8774227.
Du K, Zheng X, Ma ZT, Lv JY, Jiang WJ, Liu MY. Association of Circulating Magnesium Levels in Patients With Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front Aging Neurosci. 2021;13:799824. https://doi.org/10.3389/fnagi.2021.799824 . (Epub 2022/01/28. PubMed PMID: 35082658; PubMed Central PMCID: PMCPMC8784804).
Saitsu Y, Nishide A, Kikushima K, Shimizu K, Ohnuki K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed Res. 2019;40(4):125–31. https://doi.org/10.2220/biomedres.40.125 . (Epub 2019/08/16. PubMed PMID: 31413233).
Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother Res. 2009;23(3):367–72. https://doi.org/10.1002/ptr.2634 . (Epub 2008/10/11. PubMed PMID: 18844328).
Xiang S, Ji JL, Li S, Cao XP, Xu W, Tan L, et al. Efficacy and Safety of probiotics for the treatment of alzheimer’s disease, mild cognitive impairment, and Parkinson’s Disease: a systematic review and meta-analysis. Front Aging Neurosci. 2022;14:730036. https://doi.org/10.3389/fnagi.2022.730036 . (Epub 2022/02/22. PubMed PMID: 35185522; PubMed Central PMCID: PMCPMC8851038).
Fogelman I, West T, Braunstein JB, Verghese PB, Kirmess KM, Meyer MR, Contois JH, Shobin E, Ferber KL, Gagnon J, Rubel CE, Graham D, Bateman RJ, Holtzman DM, Huang S, Yu J, Yang S, Yarasheski KE. Independent study demonstrates amyloid probability score accurately indicates amyloid pathology. Ann Clin Transl Neurol. 2023;10(5):765–78. https://doi.org/10.1002/acn3.51763 . (Epub 2023 Mar 28. PMID: 36975407; PMCID: PMC10187729).
Exploratory data analysis. John W. Tukey, 1977. Addison-Wesley, Reading MA. https://doi.org/10.1002/bimj.4710230408 .
Zhuang Z, Yang R, Wang W, Qi L, Huang T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J Neuroinflammation. 2020;17(1):288. https://doi.org/10.1186/s12974-020-01961-8 . (PMID:33008395;PMCID:PMC7532639).
Cammann D, Lu Y, Cummings MJ, Zhang ML, Cue JM, Do J, Ebersole J, Chen X, Oh EC, Cummings JL, Chen J. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci Rep. 2023;13(1):5258. https://doi.org/10.1038/s41598-023-31730-5 . (PMID:37002253;PMCID:PMC10066300).
Borsom EM, Conn K, Keefe CR, Herman C, Orsini GM, Hirsch AH, Palma Avila M, Testo G, Jaramillo SA, Bolyen E, Lee K, Caporaso JG, Cope EK. Predicting Neurodegenerative Disease Using Prepathology Gut Microbiota Composition: a Longitudinal Study in Mice Modeling Alzheimer’s Disease Pathologies. Microbiol Spectr. 2023;11(2):e0345822. https://doi.org/10.1128/spectrum.03458-22 . (Epub ahead of print. PMID: 36877047; PMCID: PMC10101110).
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9–21. https://doi.org/10.1056/NEJMoa2212948 . (Epub 2022 Nov 29 PMID: 36449413).
Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–7. https://doi.org/10.1001/jama.280.23.2001 .
Ornish D, Weidner G, Fair WR, Marlin R, Pettengill EB, Raisin CJ, Dunn-Emke S, Crutchfield L, Jacobs FN, Barnard RJ, Aronson WJ, McCormac P, McKnight DJ, Fein JD, Dnistrian AM, Weinstein J, Ngo TH, Mendell NR, Carroll PR. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174(3):1065–9. https://doi.org/10.1097/01.ju.0000169487.49018.73 . (discussion 1069-70. PMID: 16094059).
Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, Marlin R, Frenda SJ, Magbanua MJM, Daubenmier J, Estay I, Hills NK, Chainani-Wu N, Carroll PR, Blackburn EH. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–20. https://doi.org/10.1016/S1470-2045(13)70366-8 . (Epub 2013 Sep 17 PMID: 24051140).
Kivipelto M et al. Multimodal preventive trial for Alzheimer’s disease. Alzheimer’s Dement. 2021;17(Suppl.10):e056105. https://alz-journals.onlinelibrary.wiley.com/doi/abs/10.1002/alz.056105 .
Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, McLanahan SM, Kirkeeide RL, Brand RJ, Gould KL. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129–33. https://doi.org/10.1016/0140-6736(90)91656-u . (PMID: 1973470).
Download references
Acknowledgements
We are grateful to each of the following people who made this study possible. Paramount among these are all of the study participants and their spouse or support person. Their commitment was inspiring, and without them this study would not have been possible. Each of the staff who provided and supported this program is exceptionally caring and competent, and includes: Heather Amador, who coordinated and administered all grants and infrastructure; Tandis Alizadeh, who is chief of staff; as well as Lynn Sievers, Nikki Liversedge, Pamela Kimmel, Stacie Dooreck, Antonella Dewell, Stacey Dunn-Emke, Marie Goodell, Emily Dougherty, Kamala Berrio, Kristin Gottesman, Katie Mayers, Dennis Malone, Sarah & Mary Barber, Steven Singleton, Kevin Lane, Laurie Case, Amber O’Neill, Annie DiRocco, Alison Eastwood, Sara Henley, Sousha Naghshineh, Sarah Reinhard, Laura Kandell, Alison Haag, Sinead Lafferty, Haley Perkins, Chase Delaney, Danielle Marquez, Ava Hoffman, Sienna Lopez, and Sophia Gnuse. Dr. Caitlin Moore conducted much of the cognition and function testing along with Dr. Catherine Madison, Trevor Ragas, Andrea Espinosa, Lorraine Martinez, Davor Zink, Jeff Webb, Griffin Duffy, Lauren Sather, and others. Dr. Cecily Jenkins trained the ADAS-Cog rater. Dr. Jan Krumsiek and Dr. Richa Batra performed important analyses in Dr. Rima Kaddurah-Daouk’s lab. Dr. Pia Kivisåkk oversaw biomarker assays in Dr. Steven Arnold's lab. We are grateful to all of the referring neurologists. Board members of the nonprofit Preventive Medicine Research Institute provided invaluable oversight and support, including Henry Groppe, Jenard & Gail Gross, Ken Hubbard, Brock Leach, and Lee Stein, as well as Joel Goldman.
Author’s information
DO is the corresponding author. RT contributed as the senior author.
We are very grateful to Leonard A. Lauder & Judith Glickman Lauder; Gary & Laura Lauder; Howard Fillit and Mark Roithmayr of The Alzheimer’s Drug Discovery Foundation; Mary & Patrick Scanlan of the Mary Bucksbaum Scanlan Family Foundation; Laurene Powell Jobs/Silicon Valley Community Foundation; Pierre & Pamela Omidyar Fund/Silicon Valley Community Foundation (Pat Christen and Jeff Alvord); George Vradenburg Foundation/Us Against Alzheimer’s; American Endowment Foundation (Anna & James McKelvey); Arthur M. Blank Family Foundation/Around the Table Foundation (Elizabeth Brown, Natalie Gilbert, Christian Amica); John Paul & Eloise DeJoria Peace Love & Happiness Foundation (Constance Dykhuizen); Maria Shriver/Women’s Alzheimer’s Movement (Sandy Gleysteen, Laurel Ann Gonsecki, Erin Stein); Mark Pincus Family Fund/Silicon Valley Community Foundation; Christy Walton/Walton Family Foundation; Milken Family Foundation; The Cleveland Clinic Lou Ruvo Center for Brain Health (Larry Ruvo); Jim Greenbaum Foundation; R. Martin Chavez; Wonderful Company Foundation (Stewart & Lynda Resnick); Daniel Socolow; Anthony J. Robbins/Tony Robbins Foundation; John Mackey; John & Lisa Pritzker and the Lisa Stone Pritzker Family Foundation; Ken Hubbard; Greater Houston Community Foundation (Jenard & Gail Gross); Henry Groppe; Brock & Julie Leach Family Charitable Foundation; Bucksbaum/Baum Foundation (Glenn Bucksbaum & April Minnich); YPO Gold Los Angeles; Lisa Holland/Betty Robertson; the Each Foundation (Lionel Shaw); Moby Charitable Fund; California Relief Program; Gary & Lisa Schildhorn; McNabb Foundation (Ricky Rafner); Renaissance Charitable Foumdation (Stephen & Karen Slinkard); Network for Good; Ken & Kim Raisler Foundation; Miner Foundation; Craiglist Charitable Fund (Jim Buckmaster and Annika Joy Quist); Gaurav Kapadia; Healing Works Foundation/Wayne Jonas; and the Center for Innovative Medicine (CIMED) at the Karolinska Institutet, Hjärnfonden, Stockholms Sjukhem, Research Council for Health Working Life and Welfare (FORTE). In-kind donations were received from Alan & Rob Gore of Body Craft Recreation Supply (exercise equipment), Dr. Andrew Abraham of Orgain, Paul Stamets of Fungi Perfecta ( Host Defense Lion’s Mane), Nordic Naturals, and Flora. Dr. Rima Kaddurah-Daouk at Duke is PI of the Alzheimer Gut Microbiome Project (funded by NIA U19AG063744). She also received additional funding from NIA that has enabled her research (U01AG061359 & R01AG081322).
The funders had no role in the conceptualization; study design; data collection; analysis; and interpretation; writing of the report; or the decision to submit for publication.
Author information
Authors and affiliations.
Preventive Medicine Research Institute, 900 Bridgeway, Sausalito, CA, USA
Dean Ornish, Catherine Madison, Anne Ornish, Nancy DeLamarter, Noel Wingers & Carra Richling
University of California, San Francisco and University of California, San Diego, USA
Dean Ornish
Ray Dolby Brain Health Center, California Pacific Medical Center, San Francisco, CA, USA
Catherine Madison
Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institute, Karolinska vägen 37 A, SE-171 64, Solna, Sweden
Miia Kivipelto
Theme Inflammation and Aging, Karolinska University Hospital, Karolinska vägen 37 A, SE-171 64, Stockholm, Solna, Sweden
The Ageing Epidemiology (AGE) Research Unit, School of Public Health, Imperial College London, St Mary’s Hospital, Norfolk Place, London, W2 1PG, United Kingdom
Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Yliopistonranta 8, 70210, Kuopio, Finland
Clinical Services, Preventive Medicine Research Institute, Bridgeway, Sausalito, CA, 900, USA
Colleen Kemp & Sarah Tranter
Division of Biostatistics, Department of Epidemiology & Biostatistics, UCSF, San Francisco, CA, USA
Charles E. McCulloch
Neurosciences, University of California, San Diego, CA, USA
Douglas Galasko
Clinical Neurology, School of Medicine, University of Nevada, Reno, USA
Renown Health Institute of Neurosciences, Reno, NV, USA
Harvard Medical School, Boston, MA, USA
Dorene Rentz, Rudolph E. Tanzi & Steven E. Arnold
Center for Alzheimer Research and Treatment, Boston, MA, USA
Dorene Rentz
Mass General Brigham Alzheimer Disease Research Center, Boston, MA, USA
Elizabeth Blackburn Lab, UCSF, San Francisco, CA, USA
UCSF, San Francisco, CA, USA
Departments of Medicine and Psychiatry, Duke University Medical Center and Member, Duke Institute of Brain Sciences, Durham, NC, USA
Rima Kaddurah-Daouk
Department of Pediatrics; Department of Computer Science & Engineering; Department of Bioengineering; Center for Microbiome Innovation, Halıcıoğlu Data Science Institute, University of California, San Diego, La Jolla, CA, USA
Department of Pediatrics and Scientific Director, American Gut Project and The Microsetta Initiative, University of California San Diego, La Jolla, CA, USA
Daniel McDonald
Bioinformatics and Systems Biology Program; Rob Knight Lab; Medical Scientist Training Program, University of California, San Diego, La Jolla, CA, USA
Lucas Patel
Buck Institute for Research on Aging, San Francisco, CA, USA
Eric Verdin
University of California, San Francisco, CA, USA
Genetics and Aging Research Unit, Boston, MA, USA
Rudolph E. Tanzi
McCance Center for Brain Health, Boston, MA, USA
Massachusetts General Hospital, Boston, MA, USA
Interdisciplinary Brain Center, Massachusetts General Hospital, Boston, MA, USA
Steven E. Arnold
You can also search for this author in PubMed Google Scholar
Contributions
DO, CM, MK, CK, DG, JA, DR, CEM, JL, KN, AO, ST, ND, NW, CR, RKD, RK, EV, RT, and SEA were involved in the study design and conduct. DO conceptualized the study hypotheses (building on the work of MK), obtained funding, prepared the first draft of the manuscript, and is the principal investigator. CEM oversaw the statistical analyses and interpretation, and DR oversaw the cognition and function testing and interpretation. CK and ST oversaw all clinical operations and patient recruitment, including the IRB. JL conducted the telomere analyses. CM oversaw patient selection. AO developed the learning management system and community platform for patients and providers. KN managed an IRB. ND co-led most of the support groups, and CR oversaw all aspects involving nutrition. All authors participated in writing the manuscript. NW and ST oversaw data collection and prepared the databases other than the microbiome databases which were overseen by RK and prepared by DM and LP who helped design this part of the study. CM, CK, JL, RKD, RK, DM, and LP were involved in the acquisition of data. SA, RT, and RKD did biomarker analyses. All authors contributed to critical review of the manuscript and approved the final manuscript.
Corresponding author
Correspondence to Dean Ornish .
Ethics declarations
Competing interests.
MK is one of the Editors-in-Chief of this journal and has no relevant competing interests and recused herself from the review process. RKD is an inventor on key patents in the field of metabolomics and holds equity in Metabolon, a biotech company in North Carolina. In addition, she holds patents licensed to Chymia LLC and PsyProtix with royalties and ownership. DO and AO have consulted for Sharecare and have received book royalties and lecture honoraria and, with CK, have received equity in Ornish Lifestyle Medicine. RK is a scientific advisory board member and consultant for BiomeSense, Inc., has equity and receives income. He is a scientific advisory board member and has equity in GenCirq. He is a consultant and scientific advisory board member for DayTwo, and receives income. He has equity in and acts as a consultant for Cybele. He is a co-founder of Biota, Inc., and has equity. He is a cofounder of Micronoma, and has equity and is a scientific advisory board member. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. DM is a consultant for BiomeSense. RT is a co-founder and equity holder in Hyperion Rx, which produces the flashing-light glasses at a theta frequency of 7.83 Hz used as an optional aid to meditation. The rest of the authors declare that they have no competing interests.
Ethics approval and consent to participate
This clinical trial was approved by the Western Institutional Review Board on 12/31/2017 (approval number: 20172897) and all participants and their study partners provided written informed consent. The trial protocol was also approved by the appropriate Institutional Review Board of all participating sites; and all subjects provided informed consent.
Consent for publication
Informed consent was received from all patients. All data from research participants described in this paper is de-identified.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1. , rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ornish, D., Madison, C., Kivipelto, M. et al. Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: a randomized, controlled clinical trial. Alz Res Therapy 16 , 122 (2024). https://doi.org/10.1186/s13195-024-01482-z
Download citation
Received : 21 February 2024
Accepted : 15 May 2024
Published : 07 June 2024
DOI : https://doi.org/10.1186/s13195-024-01482-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Alzheimer’s
- Lifestyle medicine
- Social support
Alzheimer's Research & Therapy
ISSN: 1758-9193
- General enquiries: [email protected]
- Supplements
The Forbes Health editorial team is independent and objective. To help support our reporting work, and to continue our ability to provide this content for free to our readers, we receive compensation from the companies that advertise on the Forbes Health site. This compensation comes from two main sources. First , we provide paid placements to advertisers to present their offers. The compensation we receive for those placements affects how and where advertisers’ offers appear on the site. This site does not include all companies or products available within the market. Second , we also include links to advertisers’ offers in some of our articles; these “affiliate links” may generate income for our site when you click on them.
The compensation we receive from advertisers does not influence the recommendations or advice our editorial team provides in our articles or otherwise impact any of the editorial content on Forbes Health. While we work hard to provide accurate and up-to-date information that we think you will find relevant, Forbes Health does not and cannot guarantee that any information provided is complete and makes no representations or warranties in connection thereto, nor to the accuracy or applicability thereof.
Health Benefits Of Lion’s Mane
Expert Reviewed
Have a question for Nancy Lovering or our other editors?
Ask here for a chance to be featured in a story.
Send a note to Nancy Lovering, Lenore Cangeloso, L.Ac. M.S.A.Om. and our other editors. We read every email.
Keep reading Forbes Advisor for the chance to see the answer to your question in one of our upcoming stories. Our editors also may be in touch with follow-up questions.

Table of Contents
What is lion’s mane mushroom, lion’s mane health benefits, may benefit brain health, does lion’s mane have side effects, how to use lion’s mane, what to look for when purchasing lion’s mane.
If you’ve ever noticed pom-pom-shaped growths on the trunks of broadleaf trees like beech or oak, it might have been a lion’s mane mushroom (hericium erinaceus). Lion’s mane mushroom grows in forests across North America, Asia and Europe.
Lion’s mane is an herb that has been used for centuries for its many medicinal purposes, says Trista Best, a registered dietician, environmental health specialist and consultant with Balance One Supplements.
Continue reading to learn more about lion’s mane mushroom, including its history of traditional use in Chinese medicine, as well as potential health benefits that encompass supporting cognition and mood and reducing anxiety and inflammation.

- Contains 1000mg of Organic Lion's Mane in every serving
- Formulated with organic reishi, lion’s mane, and Cordyceps sinensis
- Packed with bioactive constituents that promote overall health and longevity
- Informed Choice Certified for dosage, purity, and safety from banned substances
Lion’s mane is a mushroom with a history of both medicinal and culinary uses in Asia and Europe. Medicinal mushroom use dates back to 450 BCE when Greek physician Hippocrates discovered the potential anti-inflammatory properties of fungi as well as its role in wound cauterization, according to a 2017 study in the Journal of Restorative Medicine [1] Spelman K, Sutherland E, Bagade A. Neurological Activity of Lion’s Mane (Hericium erinaceus) . Journal of Restorative Medicine. 2017;6(1)19-26. .
Lion’s mane grows on old or dead broadleaf tree trunks. Broadleaf trees shed their leaves seasonally and spread their seeds using a vessel, such as fruit.
Lion’s mane is composed of two parts: the visible fruiting body (the mushroom) and the mycelium, which is the bottom structure that resembles roots. Both the fruiting body and the root-like mycelium contain compounds that offer potential health benefits.
The potential benefits of lion’s mane mushroom are numerous and span physical, cognitive and mental health [2] Ghosh S, Nandi S, et alBanerjee A, Sarkar S, Chakraborty N. Prospecting Medicinal Properties of Lion’s Mane Mushroom . Journal of Food Biochemistry. 2021;45(8). . The mushroom is a source of natural bioactive compounds, which are health-promoting chemicals found in certain foods and plants. As a result, it exhibits disease-fighting properties, including anti-cancer, anti-microbial and antioxidant activity.
Research also suggests that lion’s mane may protect nerves from disease or decline, according to a 2015 abstract in the Journal of Agricultural and Food Chemistry . The same study concludes the mushroom displays additional health-promoting benefits, such as:
- Regulates blood sugar
- Reduces high blood pressure
- Promotes healthy energy levels and combats fatigue
- Helps to prevent excess blood lipid accumulation
- Protects heart health
- Slows biological aging
- Protects liver health
- Protects kidney health
Potential Alternative Treatment for Depression
Lion’s mane mushroom may be a potential alternative treatment for depression, according to a 2020 abstract in the Journal of Molecular Science . The abstract highlights three ways in which lion’s mane may ease depression symptoms:
- Helping ensure the presence of sufficient neurotransmitters
- Reducing the loss of nerve growth brought about by stressful situations
- Minimizing inflammation linked to depression [3] Chong PS, Fung ML, Wong KH, Lim LW. Therapeutic Potential of Hericium erinaceus for Depressive Disorder . International Journal of Molecular Sciences. 2020;21(1):163. .
Furthermore, research shows that people living with major depressive disorder may have lower nerve growth factor than non-depressed people, according to a 2015 meta-analysis in Neuropsychiatric Disease and Treatment [4] Chen YW, Lin PY, et al. Significantly Lower Nerve Growth Factor Levels in Patients with Major Depressive Disorder than in Healthy Subjects: A Meta-Analysis and Systematic Review . Neuropsychiatric Disease and Treatment. 2015;11:925-933. . Nerve growth factor helps nerve cells specialize, grow and remain healthy, which are important aspects of mood regulation.
A number of studies demonstrate that lion’s mane increases nerve growth factor, according to Lexi Watson, a doctor of pharmacology, functional medicine practitioner and founder of Oakley Wellness, a practice that specializes in brain health and optimal aging.
Lion’s mane’s effect on nerve growth factor levels may enable it to help protect against disorders like Alzheimer’s disease that feature cognitive impairment.
Lion’s mane is a type of nootropic, meaning it contains compounds that improve brain health and function, according to Best.
“Some research has shown a benefit on certain measures of memory and cognitive function,” says Tod Cooperman, M.D., a dietary supplement researcher and president and founder of ConsumerLab.com, a health and nutrition product testing company. “But results have been inconsistent, and most improvements have been modest at best,” he adds.
For example, lion’s mane may be effective at improving symptoms of mild cognitive impairment, according to a placebo-controlled trial in Phytotherapy Research. In the trial, adults ages 50 to 80 took four 250-milligram powdered lion’s mane tablets three times daily for 16 weeks. Cognitive function scale testing showed that participants taking lion’s mane scored higher than the placebo group, and their cognitive ability improved with the duration of supplementation. Four weeks after discontinuing lion’s mane, their cognitive test scores decreased [5] Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial . Phytotherapy Research. 2009;23(3):367-72. .
- Science-backed formulas, clinically effective dosages
- Formulated to work synergistically to fortify your mental performance, support healthy mood, and enhance resilience to stress
- No artificial sweeteners, colors, or flavors
- Made with vitamins B6 and B12
Promotes Brain Injury Recovery
A 2021 study in Antioxidants offers some promising research of lion’s mane for people who’ve experienced traumatic brain injury (TBI). The study found that both lion’s mane mushroom and coriolus versicolor (another type of mushroom also known as turkey tail) exhibit neuroprotective effects against the inflammation and oxidative stress often associated with TBI [6] D’Amico R, Salinaro AT, et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury . Antioxidants. 2021;10(6):898. .
The neurodegeneration, or progressive breakdown of nerve cells, caused by TBI can lead to further conditions like Parkinson’s disease . Treatment with lion’s mane may reduce the impact of brain trauma and TBI complications like Parkinson’s disease.
Reduces Anxiety and Stress
Lion’s mane may help ease stress, according to Best, and a 2010 study in Biomedical Research provides some evidence to support this theory. The study examines the effects of lion’s mane on brain function and concludes that participants who ate cookies containing 0.5 grams of powdered lion’s mane (specifically the mushroom or fruiting body) for four weeks reported less anxiety than those who ate placebo cookies. The study authors theorize that the nerve growth effect of lion’s mane mushroom contributes to its anti-anxiety action.
Supports Gastrointestinal Health
Lion’s mane mushroom exhibits ulcer-inhibiting action, which research suggests may stem from its effect on the helicobacter pylori (H. pylori) bacteria. H. pylori can cause stomach issues including ulcers, according to a study in the Journal of Ethnopharmacology [7] Wang M, Konishi T, Gao Y, Xu D, Gao Q. Anti-Gastric Ulcer Activity of Polysaccharide Fraction Isolated from Mycelium Culture of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Higher Basidiomycetes) . International Journal of Medicinal Mushrooms. 2015;17(11):1055-60Wang M, Konishi T, Gao Y, Xu D, Gao Q. .
If you have a medical condition or a history of asthma or allergies, consult your doctor before you try lion’s mane.
“Lion’s mane is generally well tolerated, but the most common side effects include gastrointestinal discomfort, nausea and a skin rash,” says Dr. Watson.
If you experience side effects, discontinue lion’s mane consumption until you’ve spoken with a health care provider. Hives, swelling, diarrhea and abdominal pain were symptoms of a potentially serious allergic reaction to lion’s mane mushroom, according to a 2022 case study in Annals of Allergy, Asthma & Immunology .
Lion’s mane mushroom can be taken as a supplement form, such as in capsules or a powder, or used fresh as a culinary ingredient. When used for culinary purposes, lion’s mane mushroom has a mild flavor that allows it to blend with a variety of meals and may be used as a plant-based meat substitute or a supplemental powder stirred into coffee or tea.
Lion’s mane powder is also used in savory dishes like stew, or sweet beverages like hot chocolate. It can also be made into a tea by adding hot water to mushroom pieces or powder.
Lion’s Mane Dosage
As with any supplement, it’s important to take lion’s mane as directed by the manufacturer’s instructions and not to exceed the recommended dose unless directed to do so by a health care provider.
“Most studies have provided [participants with] about 1 gram of dried mushroom (although some have used mycelium [root-like structure] or a combination of the two [mycelium and fruiting body]) given three times daily,” says Dr. Cooperman
Dr. Watson takes a more conservative approach, recommending 250 to 500 milligrams up to three times a day with or without food. The brand she recommends, Om Organic Mushroom Nutrition, contains both mycelial biomass and the fruit body.
Discover Promising Longevity Benefits
We formulated Transparent Labs Mushroom Stack with organic reishi, lion’s mane, and Cordyceps sinensis — three of the most promising medicinal mushrooms on the planet. he mushrooms in TL Mushroom Stack are packed with bioactive constituents that promote overall health and longevity.
On Transparent Labs's Website
When purchasing lion’s mane, Dr. Cooperman recommends reading labels carefully. “Be aware that most research has focused on the portion of lion’s mane that grows above ground (the mushroom) as opposed to the part underground (the mycelium),” he says. “In our tests, we found that two out of eight lion’s mane products claim to be [made from the] mushroom but are actually mycelium, as confirmed in our testing. So a consumer needs to be sure they are getting a product that contains what they are expecting.”
Unlock The Benefits of Medicinal Mushrooms
The mushrooms in Transparent Labs's Mushroom Stack are packed with bioactive constituents that promote overall health and longevity through diverse mechanisms across multiple body systems, especially the immune, gastrointestinal, and nervous systems.
- Spelman K, Sutherland E, Bagade A. Neurological Activity of Lion’s Mane (Hericium erinaceus). Journal of Restorative Medicine. 2017;6(1)19-26.
- Ghosh S, Nandi S, et alBanerjee A, Sarkar S, Chakraborty N. Prospecting Medicinal Properties of Lion’s Mane Mushroom. Journal of Food Biochemistry. 2021;45(8).
- Chong PS, Fung ML, Wong KH, Lim LW. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. International Journal of Molecular Sciences. 2020;21(1):163.
- Chen YW, Lin PY, et al. Significantly Lower Nerve Growth Factor Levels in Patients with Major Depressive Disorder than in Healthy Subjects: A Meta-Analysis and Systematic Review. Neuropsychiatric Disease and Treatment. 2015;11:925-933.
- Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytotherapy Research. 2009;23(3):367-72.
- D’Amico R, Salinaro AT, et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants. 2021;10(6):898.
- Wang M, Konishi T, Gao Y, Xu D, Gao Q. Anti-Gastric Ulcer Activity of Polysaccharide Fraction Isolated from Mycelium Culture of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Higher Basidiomycetes). International Journal of Medicinal Mushrooms. 2015;17(11):1055-60Wang M, Konishi T, Gao Y, Xu D, Gao Q.
- Boddy L, Crockatt ME, Ainsworth AM. Ecology of Hericium cirrhatum, H. coralloides and H. erinaceus in the UK. Fungal Ecology. 2011;4(2): 163-173.
- Kunca V, Ciliak M. Habitat preferences of Hericium erinaceus in Slovakia. Fungal Ecology. 2017;27(B): 189-192.
- Friedman M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. Journal of Agricultural and Food Chemistry. 2015;63,32,7108-7123.
- Choi WS, Kim YS, Park BS, Kim JE, Lee SE. Hypolipidaemic Effect of Hericium erinaceum Grown in Artemisia capillaris on Obese Rats. Mycobiology. 2013;41(2): 94–99.
- Mori K, Kikuchi H, Obara Y, Iwashita M, Azumi Y, Kinugasa S, Inatomi S, Oshima Y, Nakahata N. Inhibitory Effect of Hericenone B from Hericium erinaceus on Collagen-Induced Platelet Aggregation. Phytomedicine. 2010;17(14):1082-5.
- Lion’s Mane Supplement: What’s the Best Way to Take Lion’s Mane?. Erbology. Accessed 11/1/2022.
- Lion’s Mane Mushroom. Drugs.com. Accessed 11/1/2022.
- Bioactive Compound. Cancer.gov. Accessed 11/20/2022.
- Identify a Broadleaf Tree. Natural Resources Canada. Accessed 11/20/2022.
- Nagano N, Shimizu K, Kondo R, Hayashi C, Sato D, Kitagawa K, Ohnuki K. Reduction of Depression and Anxiety by 4 Weeks Hericium erinaceus Intake. Biomedical Research. 2010;31(4):231-237.
- Rogers RD. Neurodegeneration and Medicinal Mushrooms. Fungi. 2017;10(3):36-40.
- Watson C, Kobernick A. Dangers at the Dinner Table - A Report of Anaphylaxis to Lion’s Mane Mushroom. Annals of Allergy, Asthma & Immunology. 2022;129(5):S147.
- Nkodo A. A Systematic Review of in-vivo Studies on Dietary Mushroom Supplementation of Cognitive Impairment. Current Developments in Nutrition. 2019;3(1):14-021-19.
- Liu JH, Li L, Shang XD, Zhang JL, Tan Q. Anti-Helicobacter Pylori Activity of Bioactive Components Isolated from Hericium Erinaceus. Journal of Ethnopharmacology. 2016;183:54-58.
- Best Multivitamins For Men
- Best Multivitamins For Women
- Best Protein Powder
- Best Whey Protein Powder
- Best Protein Powder For Weight Loss
- Best Protein Powder For Muscle Gain
- Best Protein Shakes
- Best Greens Powders
- Best Magnesium Supplements
- Best Pre-Workout
- Best Vitamin C Supplements
- Best Zinc Supplements
- Best Iron Supplements
- Best Vitamins For Energy
- Best Vitamins For Hair Growth
- Best Probiotic Supplements
- Best Collagen Powders
- AG1 (Athletic Greens) Review
- Your Guide To The Best Fish Oil Supplements
- I’m A Nutrition Scientist—Here’s Why I Take A Multivitamin
- Your Guide To Vitamin D: Benefits, Best Sources And More
Next Up In Supplements
- Best Pre-Workout Supplements
- Best Protein Powders
- Best Creatine Monohydrate Supplements
- Best Vitamin Brands
- Best Protein Powders For Weight Loss
- Best Vitamin D Supplements
More from
Support your weight loss and ignite your metabolism with gnc, your guide to the best keto supplements, 51% of gen z feel pressure to get in shape for vacations, best probiotics for ibs, according to experts, best clean protein powders of 2024, according to experts, best supplements for joint pain: what to know, 6 health benefits of ginkgo biloba, according to experts.
Information provided on Forbes Health is for educational purposes only. Your health and wellness is unique to you, and the products and services we review may not be right for your circumstances. We do not offer individual medical advice, diagnosis or treatment plans. For personal advice, please consult with a medical professional.
Forbes Health adheres to strict editorial integrity standards. To the best of our knowledge, all content is accurate as of the date posted, though offers contained herein may no longer be available. The opinions expressed are the author’s alone and have not been provided, approved or otherwise endorsed by our advertisers.

Nancy Lovering is a writer with a background in public education. She’s been writing non-fiction web content for almost a decade, covering a broad range of topics relating to health and psychology. She has bylines in Psych Central, Healthline, Greatist, Health Digest, Well Within You, Care Dash and Livestrong.

Lenore Cangeloso is a board-certified acupuncturist and herbal medicine practitioner based in Oregon. She graduated with honors from Oregon College of Oriental Medicine in 2016 and obtained her bachelors of science from Oregon State University. She is also a registered yoga Instructor with a 200-hour certification from the Kripalu Institute in Massachusetts. Cangeloso has spent many months traveling to deepen her knowledge of the human body, studying massage in Thailand and traditional crafts in Mexico and Indonesia. She is a dedicated and skilled practitioner who strives to help her patients achieve optimal states of well-being.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
The Health Benefits of Lion's Mane
Benefits, Side Effects, Dosage, and Interactions
:max_bytes(150000):strip_icc():format(webp)/Caitilin-Kelly_1000-51fc24dc524d4e00b4208047363fd064.jpg)
NomadicImagery / Getty Images
- Potential Benefits
- Side Effects
- How to Take Lion's Mane
- Precautions and Interactions
- What to Look For
- Where to Find Lion's Mane
- Consult your doctor before consuming lion's mane or any other supplement.
- Call 911 immediately if you have problems breathing, throat inflammation, or other allergy symptoms.
- Use caution if you are taking diabetes or anticoagulant/antiplatelet medications.
Lion's mane ( Hericium erinaceus ) is a type of medicinal mushroom long used in traditional Chinese medicine that's widely available fresh, dried, and as a supplement. This article discusses the potential health benefits of lion's mane.
Potential Health Benefits of Lion's Mane
So far, scientific exploration of lion's mane and its effects is limited primarily to animal-based research, test-tube studies, and small clinical trials. More research is needed to understand its potential positive and negative effects and mechanisms of action in the body. Some research, however, points to possible benefits.
Brain Function
Lion's mane has shown promise in the realm of cognitive function. For example, it had positive effects in the brains of mice in a 2023 study, enhancing memory and brain cell growth. Likewise, a small study of the mushroom's impact on cognitive function and moods in young adults hinted at faster processing times and decreased stress levels. Many other studies support the idea that lion's mane might augment the brain's performance.
Likewise, lion's mane may benefit older adults with mild cognitive impairment, according to a small 2009 study. Researchers assigned 30 older adults with mild cognitive impairment to take lion's mane extract or a placebo every day for 16 weeks. In cognitive tests at weeks eight, 12, and 16 of the study, members of the lion's mane group showed significantly greater improvements in function than members of the placebo group.
In a 2011 study, scientists examined the effects of lion's mane on brain function in mice. Results revealed that lion's mane helped protect against memory problems caused by the buildup of amyloid beta (a substance that forms the brain plaques associated with Alzheimer's disease ). Studies have also shown a possible neuroprotective effect against ischemic stroke.
Depression and Anxiety
Research to date suggests that lion's mane may help alleviate depression and anxiety. For example, a 2020 review of the literature called lion's mane "a potential alternative medicine for the treatment of depression."
Likewise, a 2021 research review detailed several studies that showed significant anti-anxiety effects. Lion's mane appears to offer "neuroprotective functions, cytotoxicity, anticarcinogenic, antidiabetic, antimicrobial, and herbicidal activities," as well.
Due to a lack of supporting research, it's too soon to recommend lion's mane for any specific health condition. If you're considering the use of lion's mane for a chronic condition, make sure to consult your physician before starting your supplement regimen. Self-treating a chronic condition with lion's mane and avoiding or delaying standard care may have serious consequences.
Possible Side Effects of Lion's Mane
Little is known about the safety of long-term use and side effects of lion's mane supplements. However, there's some concern that lion's mane may aggravate symptoms in people with allergies and asthma. Therefore, it's important to consult your physician prior to using lion's mane or any other supplement, especially if you have a history of allergies, asthma, and/or any other medical condition.
Studies on the potential benefits of lion's mane in humans have shown promise, but more research is necessary. Always consult your healthcare provider before ingesting lion's mane or any other supplement.
How to Take Lion's Mane
Lion's mane hasn't been studied enough to establish standard dosages and preparation. General guidelines follow, but always heed your physician's specific advice regarding medicines and supplements.
Lion's mane is commonly consumed in many Asian countries for medicinal and culinary purposes, but there are no consistent formulations or dosage recommendations. Follow the instructions on your package of lion's mane closely.
Always consult your doctor for specific advice on how much you should take each day.
Lion's Mane Precautions and Interactions
Avoid using lion's mane mushroom products if you're pregnant. Not enough research has been done to determine if any dosage is safe during pregnancy.
If you take diabetes medications, be aware that Lion's mane mushroom can lower your blood glucose levels too much. Keep a close eye on your readings.
Likewise, taking lion's mane along with anticoagulant/antiplatelet drugs can cause blood clotting difficulties that can result in bleeding or bruising.
Some people are allergic to lion's mane. Seek medical help immediately if you notice throat swelling, breathing trouble, or other signs and symptoms after taking lion's mane.
What to Look For When Buying Lion's Mane
Some lion's mane supplements are marketed with unsupported claims, such as weight loss, brain health improvement, and heart disease prevention. Remember that these claims have not been proven definitively, Ask your healthcare provider about lion's mane before taking it for any reason.
For example, in 2019, the Food and Drug Administration (FDA) sent a warning letter to Pure Nootropics, LLC, for making unsubstantiated claims about some of their products, including their lion's mane powder. The company was marketing the supplement as "great for brain injury recovery" and to "reduce symptoms of anxiety and depression." Since then, the company has replaced this language with the claim that the product "supports overall cognitive health," with the disclaimer that the FDA has not evaluated it and it's not intended to prevent or treat any condition or disease.
Where to Find Lion's Mane
Lion's mane is widely available in big-box stores, groceries, drugstores, and online stores. Its many forms include fresh, dried, and powdered, in capsules, teas, and more. In nature, lion's mane mushrooms grow in logs, decaying wood, and tree wounds.
Martínez‐Mármol R, Chai Y, Conroy JN, et al. H ericerin derivatives activates a pan‐neurotrophic pathway in central hippocampal neurons converging to ERK1 /2 signaling enhancing spatial memory . Journal of Neurochemistry . 2023;165(6):791-808. doi:10.1111/jnc.15767
Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial . Phytother Res. 2009;23(3):367-72. doi:10.1002/ptr.2634
Mori K, Obara Y, Moriya T, Inatomi S, Nakahata N. Effects of Hericium erinaceus on amyloid β(25-35) peptide-induced learning and memory deficits in mice . Biomed Res. 2011;32(1):67-72.
I-Chen Li, et al. Neurohealth Properties of Hericium erinaceus Mycelia Enriched with Erinacines . Neurol . 2018; 2018. doi:10.1155/2018/5802634
Chong PS, Fung ML, Wong KH, Lim LW. Therapeutic potential of hericium erinaceus for depressive disorder . IJMS . 2019;21(1):163. doi:10.3390/ijms21010163
Hericium erinaceus - A rich source of diverse bioactive metabolites . FunBiotec . 2021;1(2):10-38. doi:10.5943/FunBiotec/1/2/2
Sabaratnam V, Kah-hui W, Naidu M, Rosie David P. Neuronal health - Can culinary and medicinal mushrooms help? . J Tradit Complement Med . 2013;3(1):62-8. doi:10.4103/2225-4110.106549
Lion's Mane Mushroom - Uses, Side Effects, and More . WebMD. Updated June 28, 2021.
- MyU : For Students, Faculty, and Staff
COLLEGE of BIOLOGICAL SCIENCES
- Majors and minors
- Schedule a tour of campus
- Apply for admission
- Transfer to CBS
- Scholarships for incoming students
- Information for admitted students
- Graduate programs
- Graduate School admissions
- Funding for graduate students
- Academic advising
- Career coaching
- Student engagement
- Learning abroad
- Research opportunities
- Field courses
- Scholarships
- Dean's list
- Graduation and commencement
- Graduate student governance
- Policies, forms, and resources
- Graduate commencement
- Biochemistry, Molecular Biology and Biophysics
- Biology Teaching and Learning
- Ecology, Evolution and Behavior
- Genetics, Cell Biology and Development
- Plant and Microbial Biology
- Faculty directory
- Research at CBS
- Undergraduate research
- Cedar Creek Ecosystem Science Reserve
- Itasca Biological Station and Laboratories
- Centers and institutes
- Facilities, resources and programs
- College leadership
- Diversity, equity, inclusion, justice
- Outreach and engagement
- College directory
- Dean's office directory
- Advancement
- Human resources
- Marketing and communications
- Research & Learning Technologies
- Student Services
- BioGEN administrative center
- St. Paul Administrative Cluster
- News and events
- Award-winning faculty and staff
- Resources for faculty and staff
- Resources for postdocs
- Opportunities to engage
- Alumni awards and recognition
- News for alumni and friends
- Class notes
- Update your contact info
- Giving to CBS
- Support CBS
- Ways to give
- Donor impact
- CBS Dean's Circle
The lion’s mane has long been an iconic symbol, yet there has been no clear answer as to why lions have manes, or what function they serve. Charles Darwin was the first to suggest that the mane may be a result of sexual selection, meaning that the mane increases reproductive success. The mane may protect a male’s neck during a fight with another male, or it might signal physical condition, allowing males to assess each other’s fighting ability and females to choose superior mates.
If the mane primarily serves as a shield, the neck should be a special target of attacks or wounds to the neck should be particularly dangerous. However, wounds in adult males and in females and sub-adult males (whose neck areas are bare) were no more common on the neck than on other parts of the body nor were wounds to the neck more likely to be fatal.

So what information is conveyed by the length and coloration of the lion’s mane? And if longer, darker manes are more intimidating/attractive, why don’t all male lions have extravagant manes?
Our longterm records show that males with shorter manes had often been recently injured or sick, suggesting that mane length indicates current fighting ability. Males with darker manes had higher testosterone levels, were more likely to recover from injury, spent more time resident with prides, and had higher offspring survival. Thus, mane color appears to convey information about male aggressiveness and potential reproductive success.
Variation in a sexually selected trait generally results from the inherent costs of expressing such an elaborate physical characteristic. For example, the peacock’s tail makes the male more vulnerable to predation, and only the highest-quality males can evade predators while carrying such exaggerated tails. To determine the costs of growing a large mane, we used an infrared camera to measure the surface temperatures of male and female lions. Maned males, but not those with abbreviated manes, were hotter than females, suggesting that the mane imposes a general increase in heat sensitivity. Furthermore, males with darker manes were hotter than males with blond manes. Referring again to our longterm records, we determined that males with darker manes had higher proportions of defective sperm, and ate relatively small meals in hotter weather (increasing the ratio of foraging time to food consumption). These costs are most likely heat-related and they are burdens that only superior males can bear; for inferior males, a dark mane would inflict physiological costs that outweighed the reproductive benefits.
Thus the lion’s mane appears to be a sexually selected signal by which a male advertises his quality to other lions. Our study also highlights the importance of temperature to lion ecology and behavior—which will become increasingly relevant in the face of global climate change: the Serengeti lions are already developing lighter and shorter manes than in the past. These findings were presented by Science magazine in a paper entitled “Sexual Selection, Temperature and the Lion’s Mane” by Peyton West and Craig Packer.
FURTHER READING
- The lion’s mane
- Maneless in Tsavo
- Sexual selection, temperature, and the lions’ mane
- Sexual selection, temperature, and the lions’ mane: Supporting materials and methods
- Wounding, mortality and mane morphology in African lions
123 Snyder Hall 1475 Gortner Ave St. Paul , MN 55108 United States
3-104 MCB 420 Washington Ave. S.E. Minneapolis , MN 55455 United States
- Search Please fill out this field.
- Manage Your Subscription
- Give a Gift Subscription
- Newsletters
- Sweepstakes
- Nutrition & Diet
The Science Behind 10 Lion's Mane Mushroom Benefits, From Heart to Gut Health
Are lion’s mane mushrooms healthy or overhyped? Let's dig into the research.
Haley is a Wisconsin-based creative freelancer and recent graduate. She has worked as an editor, fact checker, and copywriter for various digital and print publications. Her most recent position was in academic publishing as a publicity and marketing assistant for the University of Wisconsin Press
Eoneren/Getty Images
Lion's mane mushroom benefits have been creating quite a buzz—and with a regal name like lion’s mane, how could they not? Whether or not you’ve heard of them, when it comes to adaptogenic, functional mushrooms , lion’s mane tops the list as one of the most popular and well-studied.
But what exactly do “adaptogenic” and “functional” mean in the context of edible fungi? The benefits touted by lion's mane mushrooms are immune support, boosting brain, heart, and gut health, and more. But are the benefits completely woo-woo, or based in actual science? Here’s what to know about the benefits of lion’s mane mushrooms, both nutritional and beyond.
What exactly are lion’s mane mushrooms?
Also known as the hedgehog mushroom, or by its scientific name Hericium erinaceus , the lion’s mane mushroom has a rich history. As a centuries-old cornerstone of traditional Chinese medicine, it was used to improve overall health and longevity. Meanwhile, in Japan, Buddhist monks used the powder of this mushroom to enhance focus during meditation. Evidence dating back to as early as 450 B.C. to ancient Greece suggests lion’s mane was utilized for its anti-inflammatory and wound-healing properties.
Getty Images
Maybe one of the most compelling benefits associated with lion's mane is that it’s considered to be a functional and adaptogenic food. Adaptogenic foods help the body to adapt to stress of any kind: physical, biological, or chemical.

Lion’s Mane Mushroom Benefits
With all of the names this mushroom has accumulated comes an even larger number of health benefits. Here are some of the top evidence-based health benefits of lion’s mane mushrooms that give it such a high reputation in the health and wellness world.
Lion's Mane Can Improve Immune Health
Speaking of immune health , hedgehog mushrooms are effective at keeping our immune systems strong and functioning optimally. This is due, in part, to their bioactive plant compounds and zinc , both of which are antioxidants that help reduce inflammation in the body and fight off disease-causing free radicals. When it comes to lion’s mane, its protein and carbohydrate content also amplifies its immune-boosting powers. One animal study found that one type of protein extracted from lion's mane was associated with modulation of the immune system through regulation of the gut microbiome in mice. While another found that certain carbs in this mushroom also stimulated our intestinal bacteria, helping to enhance the body’s cellular immune system pathways.
While more human studies are needed to confirm, emerging research also suggests that these nutritional components add up when it comes to fighting off some of the scariest of illnesses, like cancer. One in vitro and animal study, for example, found lion’s mane extract to be effective against liver, colon, and even gastric cancer cells. Further studies and reviews echo these findings when it comes to this fungus’ potential to stand up to cancer.
Lion’s Mane Helps Boost and Protect Brain Health
Where these mushrooms really shine, and what they’re most known for, is their ability to positively impact our brain health. This is primarily due to the neurotrophic factors and bioactive compounds found in this functional fungus. Neurotrophic factors are biomolecules made of protein that promote the growth and differentiation of neurons, the nerve cells in the brain that send and receive information. Lion's mane has also been associated with reduced brain inflammation, offering neuroprotective benefits. Some of these benefits include symptom improvement of sleep disorders, Alzheimer’s, and Parkinson’s disease. One animal study even found lion’s mane to assist in neurotransmission and recognition memory.
Lion’s Mane Supports Gut Health
As already mentioned above, this edible fungi is also a champion for gut health. Many of the functional mushrooms, including lion’s mane, are excellent sources of beta-glucan, a kind of soluble fiber that benefits our health in a number of ways beyond just gut health, including immune, heart, and metabolic health. It also has a prebiotic effect in the microbiome, serving as food for our healthy gut bacteria. Beyond its beta-glucan content, animal studies suggest lion’s mane may potentially be beneficial in treating inflammatory bowel disease and ulcerative colitis.
Lion’s Mane Promotes Heart Health
Lion’s mane mushrooms can even help support heart health . Similar to other soluble fibers, their beta-glucans also bind to cholesterol in the small intestine and move it through the rest of the digestive tract for disposal. This means that cholesterol literally goes down the drain instead of being absorbed into your blood. Beta-glucans are also associated with reduced blood pressure levels and together, cholesterol and blood pressure make up some of the key conditions needed for heart disease to arise. Various studies reiterate these impacts, with one in vitro showing lion’s mane’s ability to help reduce bad cholesterol (or low-density lipoprotein, LDL) levels. While another review found edible plants, including lion’s mane (though it’s technically a fungus), to help promote healthy blood clotting in humans, another contributor to heart disease if functioning improperly.
Lion’s Mane Provides Important Vitamins and Minerals
Additionally, these fungi offer quite a few vitamins and minerals including potassium, iron, and B vitamins. These nutrients combine to support healthy fluid balance, immune function, red blood cell formation, and energy metabolism.
Lion’s Mane May Help Regulate Blood Sugars
These fungi also impact metabolic health, including the ability to regulate blood sugars. While this makes sense given this mushroom’s fiber content, there’s budding research to back it up. In one animal study, lion’s mane was found to help reduce blood sugars to normal levels while also providing a protective effect on the pancreas, liver, and kidneys. Another found this mushroom to help relieve diabetic nerve pain in animal subjects.
That said, more research on human subjects is required to say definitively its ability to help people with (and without) diabetes manage their blood sugar levels and aid diabetes-related nerve pain.
Lion's Mane May Alleviate Anxiety and Depression
Early research indicates that several of the chemicals in lion's mane mushrooms boost the regeneration of brain cells and improve the performance of the hippocampus, the part of the brain that regulates emotional response.
Studies also show that individuals with depression and anxiety have lower nerve growth factor (NGF). NGF helps nerve cells regenerate and remain healthy, which are factors in mood regulation. Lion's mane mushrooms promote these factors, which indicates that it could be a potential alternative medication for depression in the future.
Lion's Mane Can Help Nervous System Recovery
Individuals who have had an injury to the brain, spinal cord, or nervous system may benefit from lion's mane mushrooms. Lion's mane has the ability to aid in the regeneration of peripheral nerves. Because of these properties, scientists are looking into using lion's mane to treat a number of illnesses and injuries, including traumatic brain injuries, stroke, multiple sclerosis, and Creutzfeldt-Jakob disease, among others.
Lion's Mane Guards Against Stomach Ulcers
As mentioned earlier, lion's mane can help with gut health and certain intestinal disorders. But it also fights against stomach ulcers through a couple of mechanisms of action. Ulcers are usually caused by one of two things: first, by overuse of NSAIDs like ibuprofen, which eat away at the mucus lining of the stomach; second, through the presence of a bacteria called H. pylori . Lion's mane has been shown to thicken the mucosal lining of the stomach, fighting against ulcers caused by NSAIDs and slowing the growth of H. pylori .
Lion's Mane Reduces Inflammation
According to the NIH, chronic inflammatory diseases are the leading cause of death worldwide. Chronic inflammation is associated with diabetes, cardiovascular disease, arthritis, allergies, COPD, autoimmune disorders, and more. Studies have shown that lion's mane mushrooms contain anti-inflammatory compounds that can lessen the impact of these conditions.
How to Take Lion’s Mane
Acknowledging that several of the studies examined above were conducted in animals, there’s enough human evidence for researchers to conclude that lion’s mane is a bonafide superfood. But how can you include it in your cooking and daily routine?
Lion’s mane is one of the few functional mushrooms that you can find in its whole form relatively readily these days, especially in specialty food stores and from local producers. You can whip up any of your favorite mushroom recipes , adding it to pastas, soups, eggs, and rice dishes. However, many feature lion’s mane in vegan “seafood” recipes as it can offer a flavor and texture reminiscent of lobster, shrimp, or crab. These mushrooms can even be steeped in hot water to make a deliciously earthy tea loaded with health benefits.
Lion’s mane can also be powdered and added to stews, soups, and gravies, not only as a way to boost the recipe’s healthfulness, but to add umami flavor. However, it can just as easily be added to smoothies, coffees, teas, and oatmeal in smaller amounts without compromising the overall flavor. There are also a variety of mushroom coffee and tea alternative brands that feature this fungus including MUD/WTR , Rasa , Ryze , and Four Sigmatic .
Supplement Safety and Tips
Otherwise, there are a number of supplements available that feature lion’s mane, including pills, powders, and tinctures. When buying supplements, however, it’s extremely important to know that while the U.S. Food and Drug Administration (FDA) monitors supplements in the country, it does not test or regulate each one for safety or purity. This means that products that are not reliable or safe are as readily available on the market for purchase, ultimately placing that risk and responsibility on the consumer.
That means it's (unfortunately) our job as consumers to do our research before buying supplements to ensure their safety and purity. There are a few organizations conducting third-party verifications to help in that process including NSF and U.S. Pharmacopeia (USP). Look for their seal of approval on any products you're considering
Side Effects and Risks of Taking Lion's Mane
Scientists are still researching the side effects and possible risks of taking lion's mane supplements. No human trials have been conducted to determine the risks and significant side effects. However, no adverse effects were observed in animal trials, even at higher doses.
If you are allergic or sensitive to mushrooms, however, you should avoid taking lion's mane. Allergic reactions can present in rashes and difficulty breathing; as a general rule, you should always speak with your doctor before adding any supplements to your diet.
Frequently Asked Questions
Yes, you can; just watch your daily intake. The recommended dosage varies by individual based on weight, metabolism, and tolerance. Supplements range widely in dosage from 250 to 2,500 mg per day, so it's crucial to consult your physician before starting a regimen.
In short, it depends on who you ask. Some supplement companies state that you'll begin to see effects within two to five days. Others say it can take up to six months to see an impact. The effects will likely depend on your metabolism and the dose that you've chosen.
Adaptogenic mushroom use has become quite popular, and as a result, there are a good amount of options to choose from. The benefits vary from species to species, so pick one based on the effects you're hoping to see. In addition to lion's mane, reishi and cordyceps are also commonly used supplements. Some supplements mix different types of mushrooms in each formulation.
Khan MdA, Tania M, Liu R, Rahman MM. Hericium erinaceus: an edible mushroom with medicinal values . Journal of Complementary and Integrative Medicine . 2013;10(1). doi:10.1515/jcim-2013-0001
Spelman K, Sutherland E, Bagade A. Neurological Activity of Lion’s Mane (Hericium erinaceus) . Journal of Restorative Medicine . 2017;6(1):19-26. doi:10.14200/jrm.2017.6.0108
Xu N, Gao Z, Zhang J, et al. Hepatoprotection of enzymatic-extractable mycelia zinc polysaccharides by Pleurotus eryngii var. tuoliensis . 2017;157:196-206. doi:10.1016/j.carbpol.2016.09.082
Prasad AS. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging . Journal of Trace Elements in Medicine and Biology . 2014;28(4):364-371. doi:10.1016/j.jtemb.2014.07.019
Diling C, Chaoqun Z, Jian Y, et al. Immunomodulatory Activities of a Fungal Protein Extracted from Hericium erinaceus through Regulating the Gut Microbiota . Frontiers in Immunology . 2017;8. doi:10.3389/fimmu.2017.00666
Sheng X, Yan J, Meng Y, et al. Immunomodulatory effects of Hericium erinaceus derived polysaccharides are mediated by intestinal immunology . Food & Function . 2017;8(3):1020-1027. doi:10.1039/c7fo00071e
Li G, Yu K, Li F, et al. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers . Journal of Ethnopharmacology . 2014;153(2):521-530. doi:10.1016/j.jep.2014.03.003
Lee SR, Jung K, Noh H, et al. A new cerebroside from the fruiting bodies of Hericium erinaceus and its applicability to cancer treatment . Bioorganic & medicinal chemistry letters . 2015. doi:10.1016/j.bmcl.2015.10.092
Blagodatski A, Yatsunskaya M, Mikhailova V, Tiasto V, Kagansky A, Katanaev VL. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy . Oncotarget . 2018;9(49). doi:10.18632/oncotarget.25660
Lai PL, Naidu M, Sabaratnam V, et al. Neurotrophic Properties of the Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia . International Journal of Medicinal Mushrooms . 2013;15(6):539-554. doi:10.1615/intjmedmushr.v15.i6.30
Kushairi, Phan, Sabaratnam, David, Naidu. Lion’s Mane Mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Suppresses H2O2-Induced Oxidative Damage and LPS-Induced Inflammation in HT22 Hippocampal Neurons and BV2 Microglia . Antioxidants . 2019;8(8):261. doi:10.3390/antiox8080261
Chong PS, Fung ML, Wong KH, Lim LW. Therapeutic Potential of Hericium erinaceus for Depressive Disorder . International Journal of Molecular Sciences . 2019;21(1):163. doi:10.3390/ijms21010163
Vigna L, Morelli F, Agnelli GM, et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evidence-Based Complementary and Alternative Medicine . 2019;2019:1-12. doi:10.1155/2019/7861297
Li I-Chen, Chang HH, Lin CH, et al. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study . Frontiers in Aging Neuroscience . 2020;12. doi:10.3389/fnagi.2020.00155
Brandalise F, Cesaroni V, Gregori A, et al. Dietary Supplementation ofHericium erinaceusIncreases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice . Evidence-Based Complementary and Alternative Medicine . 2017;2017:1-13. doi:10.1155/2017/3864340
Bashir KM, Choi JS. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future . International Journal of Molecular Sciences . 2017;18(9):1906. doi:10.3390/ijms18091906
Murphy EJ, Rezoagli E, Major I, Rowan NJ, Laffey JG. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application . Journal of Fungi . 2020;6(4). doi:10.3390/jof6040356
Chen C, Huang X, Wang H, Geng F, Nie S. Effect of β-glucan on metabolic diseases: a review from the gut microbiota perspective . Current Opinion in Food Science . 2022;47:100907. doi:10.1016/j.cofs.2022.100907
Diling C, Xin Y, Chaoqun Z, et al. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota . Oncotarget . 2017;8(49):85838-85857. doi:10.18632/oncotarget.20689
Qin M, Geng Y, Lu Z, et al. Anti-Inflammatory Effects of Ethanol Extract of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), in Mice with Ulcerative Colitis . International Journal of Medicinal Mushrooms . 2016;18(3):227-234. doi:10.1615/intjmedmushrooms.v18.i3.50
Liska DJ, Dioum E, Chu Y, Mah E. Narrative Review on the Effects of Oat and Sprouted Oat Components on Blood Pressure . Nutrients . 2022;14(22):4772. doi:10.3390/nu14224772
Choi WS, Kim YS, Park BS, Kim JE, Lee SE. Hypolipidaemic Effect of Hericium erinaceum Grown in Artemisia capillaris on Obese Rats . Mycobiology . 2013;41(2):94-99. doi:10.5941/myco.2013.41.2.94
Albadawi DAI, Ravishankar D, Vallance TM, Patel K, Osborn HMI, Vaiyapuri S. Impacts of Commonly Used Edible Plants on the Modulation of Platelet Function . International Journal of Molecular Sciences . 2022;23(2):605. doi:10.3390/ijms23020605
McLean RM, Wang NX. Potassium . Advances in Food and Nutrition Research . 2021;96:89-121. doi:10.1016/bs.afnr.2021.02.013
Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health . Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences . 2014;19(2):164-174.
Hanna M, Jaqua E, Nguyen V, Clay J. B Vitamins: Functions and Uses in Medicine . The Permanente Journal . 2022;26(2):89-97. doi:10.7812/tpp/21.204
Zhang C, Li J, Hu C, et al. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice . Scientific Reports . 2017;7(1). doi:10.1038/s41598-017-11457-w
Ryu S, Kim HG, Kim JY, Kim SY, Cho KO. Hericium erinaceus extract reduces anxiety and depressive behaviors by promoting hippocampal neurogenesis in the adult mouse brain . J Med Food. 2018;21(2):174-180.
Chong PS, Fung ML, Wong KH, Lim LW. Therapeutic potential of hericium erinaceus for depressive disorder . Int J Mol Sci. 2019;21(1):163.
Wong KH, Kanagasabapathy G, Naidu M, David P, Sabaratnam V. Hericium erinaceus (Bull. : fr.) Pers., a medicinal mushroom, activates peripheral nerve regeneration . Chin J Integr Med. 2016;22(10):759-767.
Huang HT, Ho CH, Sung HY, et al. Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein . Sci Rep. 2021;11:6551.
Woolf A, Rose R. Gastric ulcer. In: StatPearls. StatPearls Publishing; 2024.
Wang G, Zhang X, Maier SE, Zhang L, Maier RJ. in vitro and in vivo inhibition of helicobacter pylori by ethanolic extracts of lion’s mane medicinal mushroom, hericium erinaceus(Agaricomycetes) . IJM. 2019;21(1).
Pahwa R, Goyal A, Jialal I. Chronic inflammation . In: StatPearls. StatPearls Publishing; 2024.
Hou Y, Ding X, Hou W. Composition and antioxidant activity of water-soluble oligosaccharides from Hericium erinaceus . Mol Med Rep. 2015;11(5):3794-3799.
He X, Wang X, Fang J, et al. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review . International Journal of Biological Macromolecules . 2017;97:228-237. doi:10.1016/j.ijbiomac.2017.01.040
U.S. Food and Drug Administration. Dietary Supplements .
Lee LY, Li IC, Chen WP, Tsai YT, Chen CC, Tung KC. Thirteen-week oral toxicity evaluation of erinacine aenriched lion’s mane medicinal mushroom, hericium erinaceus (Agaricomycetes), mycelia in sprague-dawley rats . Int J Med Mushrooms. 2019;21(4):401-411.
Related Articles
VITAMINS & SUPPLEMENTS CENTER
- Find a Vitamin or Supplement
- Find a Vitamin by Condition
- Assess Your Vitamin Needs
DRUGS AND MEDICATIONS CENTER
- Find a Drug
- My Medicine
- Pill Identifier
- Interaction Checker
- Latest Drug News
- Find a Vitamin
- Find a Pharmacy
FIRST AID RESOURCES
- First Aid A-Z
- First Aid Kit & Wound Care
- First Aid Mobile
RELATED TO VITAMINS & SUPPLEMENTS
- Drugs & Medications
- Diet & Weight Management
- Food & Recipes
- Vitamins & Supplements
- lion's mane mushroom
LION'S MANE MUSHROOM - Uses, Side Effects, and More
Side effects.
- Precautions
- Interactions
- Reviews (27)
Uses & Effectiveness ?
We currently have no information for LION'S MANE MUSHROOM overview .
Special Precautions and Warnings
Interactions , moderate interaction.
Be cautious with this combination
Medications for diabetes (Antidiabetes drugs) interacts with LION'S MANE MUSHROOM
Lion's mane mushroom might lower blood sugar levels. Taking lion's mane mushroom along with diabetes medications might cause blood sugar to drop too low. Monitor your blood sugar closely.
Medications that slow blood clotting (Anticoagulant / Antiplatelet drugs) interacts with LION'S MANE MUSHROOM
Lion's mane mushroom might slow blood clotting. Taking lion's mane mushroom along with medications that also slow blood clotting might increase the risk of bruising and bleeding.
Medications that decrease the immune system (Immunosuppressants) interacts with LION'S MANE MUSHROOM
Lion's mane mushroom can increase the activity of the immune system. Some medications, such as those used after a transplant, decrease the activity of the immune system. Taking lion's mane mushroom along with these medications might decrease the effects of these medications.
Abdulla MA, Fard AA, Sabaratnam V, et al. Potential activity of aqueous extract of culinary-medicinal Lion's Mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) in accelerating wound healing in rats. Int J Med Mushrooms. 2011;13(1):33-9. View abstract.
Abdullah N, Ismail SM, Aminudin N, Shuib AS, Lau BF. Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evid Based Complement Alternat Med. 2012;2012:464238. View abstract.
Chang HC, Yang HL, Pan JH, et al. Hericium erinaceus Inhibits TNF-a-Induced Angiogenesis and ROS Generation through Suppression of MMP-9/NF-?B Signaling and Activation of Nrf2-Mediated Antioxidant Genes in Human EA.hy926 Endothelial Cells. Oxid Med Cell Longev. 2016;2016:8257238. View abstract.
Cheng JH, Tsai CL, Lien YY, Lee MS, Sheu SC. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement Altern Med. 2016;16(1):170. View abstract.
Cui F, Gao X, Zhang J, et al. Protective Effects of Extracellular and Intracellular Polysaccharides on Hepatotoxicity by Hericium erinaceus SG-02. Curr Microbiol. 2016 Jun 4. [Epub ahead of print] View abstract.
Grozier CD, Alves VA, Killen LG, Simpson JD, O'Neal EK, Waldman HS. Four weeks of Hericium erinaceus supplementation does not impact markers of metabolic flexibility or cognition. Int J Exerc Sci 2022;15(2):1366-1380. View abstract.
Han ZH, Ye JM, Wang GF. Evaluation of in vivo antioxidant activity of Hericium erinaceus polysaccharides. Int J Biol Macromol. 2013;52:66-71. View abstract.
Hao L, Xie Y, Wu G, et al. Protective Effect of Hericium erinaceus on Alcohol Induced Hepatotoxicity in Mice. Evid Based Complement Alternat Med. 2015;2015:418023. View abstract.
Hiwatashi K, Kosaka Y, Suzuki N, et al. Yamabushitake mushroom (Hericium erinaceus) improved lipid metabolism in mice fed a high-fat diet. Biosci Biotechnol Biochem. 2010;74(7):1447-51. View abstract.
Hou Y, Ding X, Hou W. Composition and antioxidant activity of water-soluble oligosaccharides from Hericium erinaceus. Mol Med Rep. 2015;11(5):3794-9. View abstract.
Kim SP, Kang MY, Choi YH, et al. Mechanism of Hericium erinaceus (Yamabushitake) mushroom-induced apoptosis of U937 human monocytic leukemia cells. Food Funct. 2011;2(6):348-56. View abstract.
Kim SP, Kang MY, Kim JH, Nam SH, Friedman M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J Agric Food Chem. 2011;59(18):9861-9. View abstract.
Kim SP, Moon E, Nam SH, Friedman M. Hericium erinaceus mushroom extracts protect infected mice against Salmonella Typhimurium-Induced liver damage and mortality by stimulation of innate immune cells. J Agric Food Chem. 2012;60(22):5590-6. View abstract.
Kim SP, Nam SH, Friedman M. Hericium erinaceus (Lion's Mane) mushroom extracts inhibit metastasis of cancer cells to the lung in CT-26 colon cancer-tansplanted mice. J Agric Food Chem. 2013;61(20):4898-904. View abstract.
Lai PL, Naidu M, Sabaratnam V, et al. Neurotrophic properties of the Lion's mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int J Med Mushrooms. 2013;15(6):539-54. View abstract.
Lee JS, Hong EK. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett. 2010;297(2):144-54. View abstract.
Lee JS, Min KM, Cho JY, Hong EK. Study of macrophage activation and structural characteristics of purified polysaccharides from the fruiting body of Hericium erinaceus. J Microbiol Biotechnol. 2009;19(9):951-9. View abstract.
Lee KF, Chen JH, Teng CC, et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int J Mol Sci. 2014;15(9):15073-89. View abstract.
Lee SR, Jung K, Noh HJ, et al. A new cerebroside from the fruiting bodies of Hericium erinaceus and its applicability to cancer treatment. Bioorg Med Chem Lett. 2015;25(24):5712-5. View abstract.
Li G, Yu K, Li F, et al. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J Ethnopharmacol. 2014;153(2):521-30. View abstract.
Li IC, Chang HH, Lin CH, et al. Prevention of early Alzheimer's disease by erinacine A-enriched Hericium erinaceus mycelia pilot double-blind placebo-controlled study. Front Aging Neurosci 2020 Jun 3;12:155. doi: 10.3389/fnagi.2020.00155. View abstract.
Liang B, Guo Z, Xie F, Zhao A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement Altern Med. 2013;13:253. View abstract.
Liu J, DU C, Wang Y, Yu Z. Anti-fatigue activities of polysaccharides extracted from Hericium erinaceus. Exp Ther Med. 2015;9(2):483-487. View abstract.
Liu JH, Li L, Shang XD, Zhang JL, Tan Q. Anti-Helicobacter pylori activity of bioactive components isolated from Hericium erinaceus. J Ethnopharmacol. 2016;183:54-8. View abstract.
Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother Res. 2009;23(3):367-72. View abstract.
Mori K, Kikuchi H, Obara Y, et al. Inhibitory effect of hericenone B from Hericium erinaceus on collagen-induced platelet aggregation. Phytomedicine. 2010;17(14):1082-5. View abstract.
Mori K, Obara Y, Hirota M, et al. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol Pharm Bull. 2008 Sep;31(9):1727-32. View abstract.
Mori K, Obara Y, Moriya T, Inatomi S, Nakahata N. Effects of Hericium erinaceus on amyloid ß(25-35) peptide-induced learning and memory deficits in mice. Biomed Res. 2011;32(1):67-72. View abstract.
Nagano M, Shimizu K, Kondo R, et al. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed Res 2010;31(4):231-7. View abstract.
Phan CW, Lee GS, Hong SL, et al. Hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. View abstract.
Product information for <em>Niaspan</em>. Abbott Laboratories. North Chicago, IL 60064. April 2015.
Rahman MA, Abdullah N, Aminudin N. Inhibitory effect on in vitro LDL oxidation and HMG Co-A reductase activity of the liquid-liquid partitioned fractions of Hericium erinaceus (Bull.) Persoon (lion's mane mushroom). Biomed Res Int. 2014;2014:828149. View abstract.
Rossi P, Cesaroni V, Brandalise F, et al. Dietary supplementation of lion's mane medicinal mushroom, Hericium erinaceus (Agaricomycetes), and spatial memory in wild-type mice. Int J Med Mushrooms. 2018;20(5):485-494. View abstract.
Ryu S, Kim HG, Kim JY, Kim SY, Cho KO. Hericium erinaceus extract reduces anxiety and depressive behaviors by promoting hippocampal neurogenesis in the adult mouse brain. J Med Food. 2018;21(2):174-180. View abstract.
Saitsu Y, Nishide A, Kikushima K, Shimizu K, Ohnuki K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed Res. 2019;40(4):125-131. View abstract.
Samberkar S, Gandhi S, Naidu M, et al. Lion's Mane, Hericium erinaceus and Tiger Milk, Lignosus rhinocerotis (Higher Basidiomycetes) Medicinal Mushrooms Stimulate Neurite Outgrowth in Dissociated Cells of Brain, Spinal Cord, and Retina: An In Vitro Study. Int J Med Mushrooms. 2015;17(11):1047-54. View abstract.
Tian B, Liu R, Xu T, et al. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J Sci Food Agric 2023;103(6):3050-3064. View abstract.
Tsai-Teng T, Chin-Chu C, Li-Ya L, et al. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer's disease-related pathologies in APPswe/PS1dE9 transgenic mice. J Biomed Sci. 2016;23(1):49. View abstract.
Tzeng TT, Chen CC, Chen CC, et al. The cyanthin diterpenoid and sesterterpene constituents of Hericium erinaceus mycelium ameliorate Alzheimer's disease-related pathologies in APP/PS1 transgenic mice. Int J Mol Sci. 2018;19(2). pii: E598. View abstract.
Wang K, Bao L, Qi Q, et al. Erinacerins C-L, isoindolin-1-ones with a-glucosidase inhibitory activity from cultures of the medicinal mushroom Hericium erinaceus. J Nat Prod. 2015;78(1):146-54. View abstract.
Wang M, Gao Y, Xu D, Gao Q. A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int J Biol Macromol. 2015;81:656-61. View abstract.
Wang XL, Xu KP, Long HP, et al. New isoindolinones from the fruiting bodies of Hericium erinaceum. Fitoterapia. 2016;111:58-65. View abstract .
Wong JY, Abdulla MA, Raman J, et al. Gastroprotective Effects of Lion's Mane Mushroom Hericium erinaceus (Bull.:Fr.) Pers. (Aphyllophoromycetideae) Extract against Ethanol-Induced Ulcer in Rats. Evid Based Complement Alternat Med. 2013;2013:492976. View abstract .
Wong KH, Kanagasabapathy G, Naidu M, David P, Sabaratnam V. Hericium erinaceus (Bull.: Fr.) Pers., a medicinal mushroom, activates peripheral nerve regeneration. Chin J Integr Med. 2014 Aug 26. View abstract.
Wong KH, Naidu M, David P, et al. Peripheral Nerve Regeneration Following Crush Injury to Rat Peroneal Nerve by Aqueous Extract of Medicinal Mushroom Hericium erinaceus (Bull.: Fr) Pers. (Aphyllophoromycetideae). Evid Based Complement Alternat Med. 2011;2011:580752. View abstract.
Xie XQ, Geng Y, Guan Q, et al. Influence of short-term consumption of Hericium erinaceus on serum biochemical markers and the changes of the gut microbiota: A pilot study. Nutrients. 2021;13(3):1008. View abstract.
Xu CP, Liu WW, Liu FX, et al. A double-blind study of effectiveness of hericium erinaceus pers therapy on chronic atrophic gastritis. A preliminary report. Chin Med J (Engl). 1985;98(6):455-6. View abstract.
Yang BK, Park JB, Song CH. Hypolipidemic effect of an Exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci Biotechnol Biochem. 2003;67(6):1292-8. View abstract.
Yi Z, Shao-Long Y, Ai-Hong W, et al. Protective Effect of Ethanol Extracts of Hericium erinaceus on Alloxan-Induced Diabetic Neuropathic Pain in Rats. Evid Based Complement Alternat Med. 2015;2015:595480. View abstract.
Zan X, Cui F, Li Y, et al. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int J Biol Macromol. 2015;76:242-53. View abstract.
Zhang CC, Yin X, Cao CY, et al. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg Med Chem Lett. 2015;25(22):5078-82. View abstract.
- Common Searches:
- Alpha Lipoic Acid
- Apple Cider Vinegar
- Black Cohosh
- Coenzyme Q - 10
- Glucosamine
- Red Yeast Rice
- St. John's Wort
Select a condition to view a list of vitamins
CONDITIONS OF USE AND IMPORTANT INFORMATION: This information is meant to supplement, not replace advice from your doctor or healthcare provider and is not meant to cover all possible uses, precautions, interactions or adverse effects. This information may not fit your specific health circumstances. Never delay or disregard seeking professional medical advice from your doctor or other qualified health care provider because of something you have read on WebMD. You should always speak with your doctor or health care professional before you start, stop, or change any prescribed part of your health care plan or treatment and to determine what course of therapy is right for you.
This copyrighted material is provided by Natural Medicines Comprehensive Database Consumer Version. Information from this source is evidence-based and objective, and without commercial influence. For professional medical information on natural medicines, see Natural Medicines Comprehensive Database Professional Version. © Therapeutic Research Faculty 2020.
- News & Analysis on Food & Beverage Development & Technology
Lion's mane spores 450% Google search growth
07-Jun-2024 - Last updated on 09-Jun-2024 at 23:57 GMT
- Email to a friend

The program, now in its third series, follows British celebrity car expert and presenter Jeremy Clarkson in his obstacle-laden attempt to become a successful rural farmer.
In a recent episode, first streamed in May, Clarkson cultivates hordes of lion's mane in his makeshift underground facility, and his wife suggests dehydrating and blending them into powder to sell as supplements.
She informs him: “I put it in my coffee every morning, and it’s like spearmint going through my whole head, your whole mind opens up and you’re really clear thinking and the mornings I don’t take it I really notices—it’s amazing!”
“Everyone who’s anyone takes lion's mane,” she adds.
While they fail to make a powder that can pass health and safety regulations, they surely created awareness of the fungi after the show gained more than five million views, breaking Amazon Prime Video's UK ratings.
Online pharmacy Landys Chemist noted an immediate uptick in Google search activity following the upload of the episode to the streaming platform. It noted that during the week of May 21 to 28, Google Trends showed search volumes for the phrase 'lion's mane mushroom powder' increased by 450%.
And in the following week, Google searches for "benefits of lion's mane" increased by a further 120% while "what does lion's mane do" rose by 200%.
"We've noticed a significant increase in interest in lion's mane mushroom recently, especially after its exposure on platforms like Clarkson’s Farm and TikTok. Traditionally, athletes have been early adopters of this mushroom for its cognitive and health benefits. It's encouraging to see that the broader public is now also recognizing its value."
The shrooming trend
Suppliers and brands throughout Europe have noticed this mushroom climb its way up in consumer perception.
“Lion's mane is a very trendy mushroom,” Robin Gurney, founder of Musheez, the Estonia-based organic mushroom supplement supplier, told NutraIngredients. “It’s up there with cordyceps as the two seen to be performance enhancers. Gymgoers buy into cordyceps for strength and endurance, and biohackers buy into lion’s mane for memory, cognition and focus.”
His firm, which supplies brands in countries across the continent, has witnessed 100% growth year on year for the last few years, but he has noted a distinct uptick in awareness in the last 12 months alone.
“It used to be that we were contacting brands and telling them about mushrooms and asking if these could be incorporated into their portfolio. Whereas now, brands are coming to us already knowing the benefits and wanting to add these to their range,” he added.
“I do think lion's mane is on the tip of everyone’s tongue," said Sophie Barrett, an independent medical herbalist and consultant for mushroom supplement brand Hifas de Terra. "It has taken off for us. Reishi used to be the number one best seller, and now it's concentrated extract of lion's mane.”
Barrett runs her own naturopathic clinic and hosts education sessions with health professionals for Hifas de Terra. She has witnessed the change in focus of these discussions as the fungi are taken increasingly seriously, not only by gen Zers and millennials but as a serious solution for health aging.
“It’s not just for young people who want to take it for their exams, but it’s for the elderly and those looking at preventative health solutions,” she said. “It’s something we’ve noticed at my clinic also. While we used to have a lot of mainly young females and a lot of people with autoimmune diseases, now we have more elderly patients.”
She added that there has been more interest coming from health practitioners beyond nutritionists, dietitians, herbalists and kinesiologists, with doctors and general practitioners starting to ask questions about how these ingredients can support their patients.
"It's great that they can see the benefits of adding these products to their patient care toolbox," she said.
Rhysa Phommachanh, head of digital at Landys Chemist, outlines the benefits of lion’s mane:
Cognitive Function
Lion’s mane mushrooms contain compounds such as hericenones and erinacines, which stimulate nerve growth factor production and, in turn, support brain health, memory and focus.
Research indicates it may have neuroprotective properties due to its ability to promote nerve growth and repair, which could potentially aid in the prevention or management of neurological conditions such as Alzheimer’s, dementia and Parkinson’s disease.
Mental Health support
Evidence suggests that consuming lion's mane mushrooms can help alleviate symptoms of stress , low mood and anxiety due to their anti-inflammatory properties. This mushroom also supports the production of nerve growth factor (NGF), which may help regulate mood and promote overall mental well-being.
Immune System Boost
The fungi contains polysaccharides, specifically beta-glucans—compounds that can help enhance the immune system.
Anti-inflammatory and antioxidant properties
Rich in antioxidant and anti-inflammatory compounds, these mushrooms can help reduce oxidative stress and inflammation.
Digestive health
Lion’s mane can promote the growth of beneficial gut bacteria and reduce inflammation in the gut lining. This can potentially aid with conditions like ulcers and inflammatory bowel syndrome.
Menopausal symptom relief
Preliminary research has indicated that supplementing lion’s mane mushrooms could provide symptomatic relief for women going through menopause, such as sleep disturbance and mood swings. This is potentially due to its beneficial effect on NGFs and cognitive enhancement.
- Culinary Inspiration for future protein products Griffith Foods | Download Product Brochure
- Sustainable sourcing strategies for business continuity Symrise | Download White Paper
- Your partner in plant-based meat alternatives ADM | Download White Paper
- TRANSITION FROM HOT FILL TO SUSTAINABLE ASEPTIC PACKAGING Husky Injection Molding Systems Ltd. | Download Insight Guide
- Enhance Your Product Line with Non-GMO Lecithin ADM | Download Product Brochure
- Case Study: Make Your Meat Go Further ADM | Download Case Study
Upcoming editorial webinars
- 24 Sep 2024 Tue Webinar Decarbonising Supply Chains at the Source
- 25 Sep 2024 Wed Webinar Eco-friendly Production from Farm to Fork
- 26 Sep 2024 Thu Webinar New Tech and Novel Ingredients for a Sustainable Future
On-demand webinars
- Unlocking Consumer Emotions: A Comprehensive Framework for Emotional Associations in Food and Beverage Product Development
- Real-time Brix monitoring - the secret ingredient your process needs for quality and efficiency
- Sweet Solutions: Exploring the Future of Sugar Reduction
- Free-From Webinar
- Food for Kids Webinar
- Personalised Nutrition: Tapping into data for healthier diets Webinar

FoodNavigator
- Advertise with us
- Press Releases – Guidelines
- Apply to reuse our content
- Contact the Editor
- Report a technical problem
- Whitelist our newsletters
- Why Register
- Editorial Calendar
- Event Calendar
- Expert Advisory Panel
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Behav Sci (Basel)

Lion’s Mane ( Hericium erinaceus ) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model
Associated data.
The data presented in this study are available upon request from the corresponding author, without undue reservation.
Alzheimer’s disease (AD) significantly impairs the life of an individual both cognitively and behaviorally. Tau and beta-amyloid (Aβ) proteins are major contributors to the etiology of AD. This study used mice modeling AD through the presence of tau pathology to assess the effects of Hericium erinaceus ( H. erinaceus ), also known as Lion’s mane, on cognitive and non-cognitive behaviors. Despite neurocognitive and neurobiological effects of H. erinaceus being seen in both healthy and transgenic mice, no research to date has explored its effects on mice with solely tau pathology. In this study, mice were placed on a diet supplemented with H. erinaceus or a standard rodent diet for 4.5 months in order to determine the effect of this medicinal mushroom on behavior. Tau mice given H. erinaceus had significantly shorter latencies to enter the center of the open field (OF) ( p < 0.05) and spent significantly more time in the open arms of the elevated zero maze (EZM) ( p < 0.001) compared to tau control mice. Mice given H. erinaceus spent significantly more time in the open arms of and made more head dips in the elevated zero maze (EZM) ( p < 0.05). While H. erinaceus had anxiolytic effects, no improvements were seen in spatial memory or activities of daily living. These findings provide additional support for the anxiolytic effects of H. erinaceus and point to its potential benefit as a therapeutic for anxiety in AD.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that negatively affects memory, language, problem-solving, consciousness, reasoning, and everyday living. AD is characterized by the unchecked accumulation of two proteins: the microtubule-associated protein tau and beta-amyloid (Aβ). β-amyloid clumps together and forms plaques in extracellular spaces between neurons, resulting in synapse loss [ 1 ]. Tau proteins normally support a healthy neuron by stabilizing microtubules [ 2 ]. Neurofibrillary tangles, or tau tangles, begin to form from these tau proteins after they have become hyperphosphorylated and dissociate from microtubules [ 3 ]; this leads to destabilization of the microtubule network and impairs neuronal communication [ 4 ]. In the AD brain, amyloid plaques and tau tangles are strongly associated with the development and progression of AD.
Treatments for AD only provide some improvements in the life of the individual that may slow the progression of the disease but do not terminate it. Investigation into treatments that may play a potential preventative role are warranted, since no new pharmacological treatments have been approved for AD since 2003 [ 5 , 6 ]. There is an urgent need for treatment to become available; however, the lack of understanding of AD, timely clinical trials, and expensive research [ 7 ] have led to the high failure rate of candidate disease-modifying treatments (DMTs) [ 8 ]. Lifestyle factors, such as diet, are more easily managed and implemented into a daily routine by individuals compared to pharmacological treatments. Mushrooms are easily accessible and do not have adverse side effects shown by prescription drugs [ 5 ]. The medicinal mushroom, Hericium erinaceus ( H. erinaceus ), has been shown to have numerous beneficial neurocognitive effects which may play a protective role against the development of AD.
H. erinaceus is an edible mushroom that is highly valued due to its various health benefits [ 9 ]. This medicinal mushroom has been shown to have many neurocognitive and mental benefits, and leads to improvements in the immune system due to its constituents hericenones and erinacines [ 5 , 10 , 11 ]. Hericenones (aromatic compounds with molecular weights between 330.4 and 598.9 g/mol [ 12 ]) and erinacines (diterpenoids with molecular weights between 360.5 and 506.6 g/mol [ 12 ]) are compounds that can be isolated from the fruiting bodies of mushrooms and cross the blood brain barrier (BBB) [ 13 , 14 ]. Their ability to cross the BBB is what allows H. erinaceus to have a high therapeutic potential. Additional benefits of H. erinaceus include the promotion of nerve growth factor (NGF) [ 15 ], promotion of brain-derived neurotrophic factor (BDNF), improvement of cognitive function [ 11 ], promotion of anti-inflammation, reduction in astrocyte activation, hippocampal neurogenesis, glial cell activation [ 16 ], reduction in nitric oxide (NO) production in BV2 microglia, and improvements in AD-related behaviors such as burrowing and nesting in mice [ 9 ] which can be viewed as analogous to activities of daily living in humans. H. erinaceus has also been shown to lessen anxiety and depression after four weeks of consumption in human subjects [ 17 ]. This is important since the onset of AD results in non-cognitive neuropsychiatric symptoms such as anxiety and depression which negatively affect the quality of life of the individual living with AD and their caregivers [ 18 ]. Thus, H. erinaceus possesses many beneficial qualities that may help combat dementia and AD [ 15 ].
Brandalise and colleagues (2017) [ 11 ] assessed the effects of H. erinaceus in wild-type (WT) mice. This was the first study to highlight the beneficial properties of H. erinaceus on healthy non-transgenic mice. One-month-old mice were administered a diet with 5% “Micotherapy Hericium” (0.025 g/g body weight) for two months. Researchers found both behavioral and biochemical effects of H. erinaceus administration. Mice receiving H. erinaceus had improved recognition memory and exploratory behavior in the novel object recognition task; this increased exploratory behavior toward novel objects was indicative of lower levels of anxiety [ 11 ]. Electrophysiological results also showed decreases in the stimulation failure rate of mossy fiber-CA3 neurons in the hippocampus when creating excitatory currents; these neurons are targeted in the neural pathway involving the perirhinal cortex, lateral entorhinal cortex, and dentate gyrus when the mice encounter novel objects [ 11 ]. This research supports the notion that H. erinaceus has beneficial effects on cognition and brain regions implicated in AD.
Researchers have also found that H. erinaceus can reduce Aβ plaque formation in the APPswe/PS1dE9 mouse model receiving erinacine A-enriched H. erinaceus Mycelia (HE-My) for 30 days [ 16 ]. Erinacines have been shown to stimulate the production of nerve growth factor (NGF) and offer neuroprotective properties [ 5 , 19 ]. In a series of biochemical assessments, HE-My administration presented multiple benefits, including: a reduction in the non-compact structure of plaques (the soluble form of Aβ plaques), an increase in insulin-degrading enzyme (IDE—an Aβ-degrading enzyme) in the cortex, and an increase in hippocampal neurogenesis. Tsai-Teng and colleagues (2016) [ 16 ] also found that continued administration of HE-My in mice from 30 days until 81 days improved burrowing and nesting behaviors in Activities of Daily Living (ADL) assessments.
H. erinaceus has also been shown to have beneficial effects on human subjects with mild AD. Li et al. (2020) [ 5 ] conducted a 1-year human pilot study with 49 eligible participants who consumed 350 mg capsules with 5 mg/g of erinacine A three times a day. Researchers found improvements in the blood biomarkers calcium, albumin, hemoglobin (Hb), superoxide dismutase (SOD), BDNF, and homocysteine (Hcy) [ 5 ]. Participants consuming the H. erinaceus mycelium capsules also exhibited improvements in APOE4, alpha-ACT (α-ACT), reductions in β-amyloid, and significant improvements in the Mini-Mental State Examination (MMSE) and Instrumental Activities of Daily Living (IADL), representing improved cognition and higher levels of independence [ 5 ]. Additionally, H. erinaceus has been shown to reduce anxiety and depression after four weeks in healthy female human participants [ 17 ]. These participants consumed H. erinaceus cookies containing 0.5 g of powdered fruiting body from the mushroom four times a day at any time of the day [ 17 ]. Researchers saw improvements as a result of H. erinaceus with the use of two scales: the Indefinite Complaints Index (ICI), an anxiety measure and a common scale used to measure the clinical effects of treatments, and the Center for Epidemiologic Studies Depression Scale (CES-D), a scale used to measure symptoms of depression by a self-report method [ 17 ]. Given the positive effects H. erinaceus has had on both healthy and cognitively impaired individuals, more research is warranted to continue exploring these benefits.
Previous studies have only assessed H. erinaceus supplementation in amyloid mouse models rather than tau [ 15 , 16 , 20 ]. As tau is a major constituent of AD pathology along Aβ plaques, it is important to assess the effects of H. erinaceus on a mouse model solely containing tau.
In the present study, the rTg4510 tau mouse model [ 21 ] was used to study the effects of H. erinaceus administration on cognitive and non-cognitive behaviors. This specific mouse model contains the P301L tau mutation; tau expression is driven in the forebrain as a result of the CamKIIa promoter system [ 21 ]. These mice show cognitive and behavioral impairments by four months of age and over time, behavior, tau accumulation, and brain atrophy worsen. Other tau mouse models, such as the JNPL3 have the same P301L tau mutation; however, they result in motor abnormalities which can impact behavioral assessments [ 22 ]. As we were interested in assessing whether H. erinaceus administration could rescue behavioral deficits in mice containing tangle pathology, this model was selected. Four total groups were evaluated in this study: tau mice on/off H. erinaceus and WT mice on/off H. erinaceus . Mice on the mushroom diet were supplemented with H. erinaceus through wet food. Mice underwent behavioral tests assessing general locomotor activity, anxiety-like and risk-taking behavior, and spatial cognition and memory. Additionally, activities of daily living (ADL), including burrowing and nesting were measured. It was hypothesized that H. erinaceus would decrease anxiety-like behaviors, increase locomotor activity, decrease deficits in spatial memory, and improve performance in ADL measures.
2. Materials and Methods
All procedures were approved by the Angelo State University Institutional Animal Care and Use Committee (IACUC) (approved protocol #21-204) and were in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
2.1. Animals
Experimental mice were bred by pairing female Fgf14 Tg(tetO-MAPT*P301L)4510Kha (tau) mice and male Tg(Camk2a-tTA)1Mmay (CaMKIIa promoter) mice (The Jackson Laboratory, Bar Harbor, ME, USA) in 2F:1M breeding harems. Tail snips from offspring were collected between days 11 and 14 and sent to Transnetyx, Inc. (Cordova, TN, USA) to appropriately genotype each animal. Offspring were weaned between 21 and 28 days old. Mice were then housed in separate cages (Animal Care Systems, Centennial, CO, USA) based on their sex, diet condition, and genotype ( Table 1 ).
Experimental Sample Sizes (Behavioral Analysis).
| Diet | Tau | Wildtype (WT) | |
|---|---|---|---|
| 12F, 13M | 8F, 8M | 20F, 21M | |
| Control Diet | 7F, 10M | 7F, 10M | 14F, 20M |
2.2. Housing
Mice were housed in an Optimice® caging system (Animal Care Systems, Centennial, CO, USA); each cage contained sani-chip bedding and was equipped with a nylabone for mice to chew and a nesting square. No more than three mice were housed in any single cage. All mice were maintained on a 12-h light/dark cycle (08:00–20:00 lights on). Food and water were provided ad libitum .
2.3. Dietary Supplementation
At four weeks of age, mice in the mushroom dietary condition began to receive Lion’s Mane Mushroom Mycelium Powder that was mixed into wet food (Host Defense Mushrooms, Fungi Perfecti, LLC., Olympia, WA, USA)); this continued throughout the duration of the study for four months. Each cage in each of the groups was administered 150 g of wet food and 100 g of standard dry food pellets. Wet food was made by combining laboratory water and dry food, waiting for the dry pellets to soften, and mixing by hand. WT and tau mice supplemented with H. erinaceus received 1/3 teaspoon of powder containing 1000 mg of Lion’s Mane and approximately 550 mg of ( H. erinaceus ) mycelium polysaccharides mixed into the 150 g of wet food. WT and tau control mice received 150 g of wet food with no H. erinaceus powder. All mice were provided food ad libitum and weights of the wet food along with the dry food were taken every four days to measure average food consumption.
2.4. Measures and Behavioral Testing
2.4.1. body weights and food consumption weights.
Body weights were collected throughout the duration of the experiment every eight days (after every second food weight collection) to assess weight gain as a result of maturation and dietary condition. Food weights were collected every four days to verify that mice were consuming a sufficient amount of wet food in accordance with their experimental condition, in comparison to the dry food provided ad libitum . Due to group housing of mice, the amount of wet food and dry food consumed was calculated by weighing the amount of wet food and dry food remaining in the cage after four days, subtracting that from the original amount (150 g for wet food; 100 g for dry food), and dividing that total by the number of mice in the cage to yield an average.
2.4.2. Open Field Test (OFT)
The Open Field Test (OFT) consists of a square enclosure that is used to assess general locomotor activity in rodents, exploratory behavior, mood, and anxiety [ 23 ]. The enclosure was a white plastic box that measures 45 × 45 × 40 cm. Mice were given a single five-minute trial while an overhead camera with SMART animal behavior software (Panlab, Harvard Apparatus, Holliston, MA, USA) measured the following variables: distance traveled in the center of the OFT (cm), percent time spent in the center, and latency (s) to first enter into the center of the box. The OFT box was cleaned with 70% ethanol between mice to eliminate olfactory cues. The number of fecal boli was also counted manually at the end of each trial before cleaning.
2.4.3. Elevated Zero Maze (EZM)
The Elevated Zero Maze (EZM) is a behavioral test measuring anxiety-like behavior and approach/avoidance conflict in rodents [ 24 ]. It is elevated off the ground and consists of an elevated ‘0’ shaped platform with two enclosed arms and two open arms on opposite ends [ 25 ]. The mouse moves between the enclosed and open arms within a single five-minute trial. Mice were considered to be inside a given arm when all four paws were in that particular arm. Mice displaying higher levels of activity by exploring the open and exposed arms of the EZM are perceived as less anxious in comparison to those who spend more time in the enclosed and protected arms [ 24 ]. The following variables were measured: number of arm transitions, time spent in the open versus closed arms, and head dips assessing risk-taking behavior [ 26 ]. Between each mouse, the EZM apparatus was cleaned with 70% ethanol to eliminate olfactory cues. One mouse was removed from EZM data analysis due to falling off the maze.
2.4.4. Morris Water Maze (MWM)
The Morris Water Maze (MWM) is a behavioral test of spatial memory where rodents must use visual cues to locate a clear platform hidden just below opaque water [ 27 ]. During a five-day acquisition period, mice were run through three trials per day. Visual cues were placed onto a curtain hanging outside of the tub. These cues aid the mice in learning the location of the hidden platform which remains stationary during the test. Mice were placed into the MWM facing the wall and were given 60 s to find the hidden platform. If the mouse did not find the platform in the allotted time, the mouse was guided towards the platform and placed there for 10 s. Each day, mice were run through a different sequence of three cues; the sequence was the same for each mouse. During each trial, the following variables were measured: percent time spent in the target quadrant, latency to reach the platform (s), thigmotaxicity (time (s) spent swimming along the border of the pool), and total distance swam (cm). An overhead camera connected to SMART animal behavior tracking software (Panlab, Harvard Apparatus, Holliston, MA, USA) recorded these variables. On day six, after the five 3-trial acquisition days, each mouse underwent a single probe trial where the platform was lowered for the animals to be unable to access it. This is meant to measure long-term memory. Mice began at a novel location (between two of the previous start locations). Target crosses when the platform was submerged, time spent in the target quadrant, and thigmotaxicity were measured on this probe day.
2.4.5. Activities of Daily Living: Burrowing and Nesting
Activities of Daily Living (ADL) are common measures for studying noncognitive aspects of AD in rodents; these primarily include burrowing and nesting. These assess the tendency for mice to have normal activity levels and general animal welfare [ 28 ]. Burrowing was analyzed first. Burrowing was assessed by individually housing mice in a shoebox cage (Ancare, Bellmore, NY, USA) containing a PVC tube with one end closed, containing 250 g of pea-gravel (small rocks). The amount of removed pea gravel was measured after 2-h and the following morning. One mouse was removed from both ADL measures due to a water bottle spilling in the shoebox cage during the 2-h burrowing assessment, which could have potentially served as a stressor to the mouse and affected performance in the ADL measures.
Once the two burrowing measures were recorded, fresh sani-chip bedding was given to each cage along with 2.5 g of shredded white paper, which was sprinkled into the cage. The following morning, pictures were taken of each cage and its nest for scoring by a rater blind to condition. Two raters rated each nest on a scale of 1–5, with 1 being no nest was constructed and 5 being a complete nest was constructed [ 29 ]. After reliability in ratings was assessed, the scores were averaged and used for analysis.
2.5. Statistical Analysis
A 2 (genotype) × 2 (diet) × 2 (sex) × 5 (time) mixed ANOVA was run to assess changes in body weights overtime and a 2 (genotype) × 2 (diet) × 2 (sex) × 4 (time) mixed ANOVA was run to assess differences in the consumption of dry and wet food over the course of the experiment.
Dependent variables in the OFT, EZM, and ADL measures (burrowing and nesting) were assessed through 2 (genotype) × 2 (diet) × 2 (sex) factorial ANOVAs.
A 2 (genotype) × 2 (diet) × 2 (sex) × 5 (acquisition days of the MWM paradigm) mixed ANOVA was run for the following dependent variables in the MWM: percent time spent in the target quadrant, latency to reach the platform (s), total distance (cm) and thigmotaxicity. Target crosses on the final probe trial (when the platform was submerged), thigmotaxicity, and time spent in the target quadrant were measured with a 2 (genotype) × 2 (diet) × 2 (sex) factorial ANOVA.
Pairwise comparisons were assessed following significant main effects and simple effects analyses were run following any significant interactions with Bonferroni corrections. Greenhouse Geiser corrections were applied when sphericity was violated in mixed ANOVA analyses. p < 0.05 was considered statistically significant and p < 0.10 was considered trending. All analyses were run through SPSS v.26 (IBM Corp., Armonk, NY, USA) and graphs were made through GraphPad Prism (v.9.3.1) (San Diego, CA, USA).
3.1. Animal Body Weights
There was a significant effect of day, F (1.679, 112.507) = 161.575, p < 0.001, η p 2 = 0.707; as the experiment progressed, mice gained weight ( Figure 1 A). There was also a significant day × diet × sex × genotype interaction, F (1.679, 112.507) = 3.745, p < 0.05, η p 2 = 0.053. In male WT mice, those given the control diet (no H. erinaceus in wet food) weighed significantly more than their mushroom counterparts only at 64 days ( p < 0.05) and 96 days ( p < 0.05) ( Figure 1 B). In female tau mice, those on the control diet weighed significantly more at each weighing time (baseline: p < 0.01; 32 days: p < 0.01; 64 days: p < 0.001; 96 days: p = 0.001; 128 days: p < 0.05) than those given H. erinaceus ( Figure 1 C). Finally, in control diet mice, female tau mice weighed significantly more than female WT mice at days 64 ( p = 0.01), 96 ( p < 0.05), and 128 ( p < 0.05) ( Figure 1 D).

Weights over time. ( A ) Mice on the control diet (no H. erinaceus ) weighed significantly more than those given H. erinaceus . ( B ) Male WT mice given the control diet weighed significantly more than their H. erinaceus counterparts at 64 days and 96 days; only male mice are graphed. ( C ) Female tau mice on the control diet weighed significantly more at each weighing point than female tau mice given H. erinaceus ; only female mice are graphed. ( D ) Female tau mice weighed significantly more than female WT mice. Female tau mice weighed more than female WT mice at days 64, 96, and 128; only control diet mice are graphed. 0 = baseline; error bars represent mean ± SEM. (* p < 0.05, ** p < 0.01, *** p < 0.001).
There was a significant between-subject effect of diet, F (1, 67) = 5.833, p < 0.05, η p 2 = 0.08; control diet mice weighed more than those given H. erinaceus ( Figure 1 A). There was also a significant diet × sex × genotype interaction, F (1, 67) = 10.291, p = 0.002, η p 2 = 0.133. In male mice, WT controls weighed significantly more than WT mushroom mice, p < 0.05 ( Figure 1 B). In female mice, tau mushroom mice weighed significantly less than tau control mice ( p = 0.001) ( Figure 1 C). In control mice (no H. erinaceus ), female tau mice weighed significantly more than female WT mice, p < 0.05 ( Figure 1 D).
3.2. Wet Food Consumption
There was a significant effect of time, F (2.689, 180.133) = 11.203, p < 0.001, η p 2 = 0.143, indicating that mice ate more wet food over the course of the experiment ( Figure 2 A). There were also significant time × diet × sex, F (2.689, 180.133) = 4.511, p < 0.01, η p 2 = 0.063, and time × diet × genotype, F (2.689, 180.133) = 3.831, p < 0.05, η p 2 = 0.054 interactions. Male control diet mice ate more wet food at 64 ( p < 0.01), 96 ( p < 0.001), and 128 ( p < 0.01) days than male mushroom mice ( Figure 2 B). Female mice given H. erinaceus ate more than male mice given H. erinaceus at 64 ( p < 0.05) and 96 ( p < 0.01) days ( Figure 2 C). Tau mice given the control diet ate more than tau mice given H. erinaceus at 32 ( p < 0.05) and 128 ( p < 0.01) days ( Figure 2 D).

Wet food consumption over time. ( A ) Mice on the control diet (no H. erinaceus ) ate more food than those given H. erinaceus . ( B ) Male control diet mice ate more wet food at day 64 ( p < 0.01), 96 ( p < 0.001), and 128 ( p < 0.01) than male mushroom mice. Tau control mice ate significantly more wet food than tau mushroom mice; only male mice graphed. ( C ) Female mice ate more than male mice at 64 ( p < 0.05) and 96 ( p < 0.01) days. Female tau mice ate significantly more than male tau mice, p < 0.001. Tau female mice ate significantly more wet food than WT female mice ( p = 0.001). WT male mice ate significantly more wet food than Tau male mice, p = 0.001; only H. erinaceus diet condition graphed. ( D ) Tau control mice ate more than tau mushroom mice at day 32 ( p < 0.05) and 128 ( p < 0.01). Female mice ate significantly more than male mice, p < 0.05. Male control mice ate significantly more wet food than male mushroom mice, p < 0.001. Female mushroom mice ate significantly more wet food than male mushroom mice, p < 0.001; only tau mice graphed. Error bars represent mean ± SEM.
There were significant effects of diet, F (1, 67) = 6.251, p < 0.05, η p 2 = 0.085, and diet × sex, F (1, 67) = 8.399, p < 0.01, η p 2 = 0.111, genotype × sex, F (1, 67) = 9.179, p < 0.01, η p 2 = 0.120, and diet × sex × genotype, F (1, 67) = 13.792, p < 0.001, η p 2 = 0.171 interactions. Control mice ate significantly more wet food than those on the H. erinaceus diet, p < 0.05 ( Figure 2 A). Female mice given mushrooms ate significantly more than males given mushrooms, p < 0.05 ( Figure 2 C). Female tau mice ate significantly more than male tau mice, p < 0.05 and tau females ate significantly more wet food than WT females, p < 0.05. Male tau mice given the control diet ate significantly more than male tau mice given H. erinaceus , p < 0.001 ( Figure 2 B). Female tau mushroom mice ate significantly more than male tau mushroom mice, p < 0.001 ( Figure 2 C). Interestingly, wet food consumption in genotype was dependent on sex in the mushroom condition: tau female mushroom mice ate significantly more than WT female mushroom mice, p = 0.001 while WT male mushroom mice ate significantly more than tau male mushroom mice, p = 0.001 ( Figure 2 C).
3.3. Dry Food Consumption
There was a significant effect of time, F (1.795, 120.248) = 8.314, p = 0.001, η p 2 = 0.110; mice ate less dry food as the experiment progressed. There was a significant sex × genotype interaction, F (1, 67) = 4.497, p < 0.05, η p 2 = 0.063. Male tau mice ate significantly more dry food than male WT mice ( p < 0.05) ( Figure 3 ).

Dry food consumption over time. As the experiment progressed, mice ate less dry food, p < 0.01. Male tau mice consumed significantly more dry food than male WT mice ( p < 0.05). Error bars represent mean ± SEM.
3.4. Open Field Test
3.4.1. latency to enter the center.
There was a significant diet × genotype interaction, F (1, 67) = 7.650, p < 0.01, η p 2 = 0.102. Simple effects analysis revealed that tau control mice had significantly longer latencies to enter the center of the OF compared to tau mice given H. erinaceus ( p < 0.05) and WT control mice ( p < 0.01) ( Figure 4 A). Analysis also revealed a trending diet × sex interaction, F (1, 67) = 3.217, p = 0.077, η p 2 = 0.046. Female mushroom mice (M = 8.630 s, SD = 11.875) took less time to enter the center of the OF than female control mice (M = 17.897 s, SD = 20.574). Male mushroom mice (M = 14.813 s, SD = 19.129) took longer to enter the center of the OF than male control mice (M = 10.216 s, SD = 16.930).

Open field test measures. ( A ) Tau control mice had significantly longer latencies to enter the center compared to WT control mice. Tau mice supplemented with H. erinaceus had significantly shorter latencies in entering the center of the OF compared to tau control mice. ( B ) Male mice defecated more than females. Error bars represent mean ± SEM (* p < 0.05, ** p < 0.01).
3.4.2. Total Distance in the Center (cm)
There was a trending effect of diet, F (1, 67) = 2.873, p < 0.10, η p 2 = 0.041. Mice in the mushroom diet (M = 1033.89 cm, SD = 1534.67) traveled a greater total distance in the center of the OFT compared to mice in the control group (M = 480.23 cm, SD = 522.65).
3.4.3. Percent Time Spent in the Center
There were no significant effects of diet, F (1, 67) = 2.464, p = 0.121, η p 2 = 0.035, sex, F (1, 67) = 0.001, p = 0.979, η p 2 = 0.000, or genotype, F (1, 67) = 0.085, p = 0.771, η p 2 = 0.001 for percent time spent in the center of the OF.
3.4.4. Fecal Boli
Analysis of fecal boli revealed a significant effect of sex, F (1, 67) = 10.227, p = 0.002, η p 2 = 0.132. Males left significantly more fecal boli than females during their time in the OFT ( p < 0.01) ( Figure 4 B). There were no significant effects of genotype, F (1, 67) = 0.049, p = 0.825. There were no differences between tau mice (M = 1.181, SD = 1.929) and WT mice (M = 1.283, SD = 2.162). Additionally, there were no significant effects of diet, F (1, 67) = 0.177, p = 0.734. There were no differences between mice administered H. erinaceus (M = 1.153, SD = 1.767) and mice consuming the control diet (M = 1.311, SD = 2.311).
3.5. Elevated Zero Maze
3.5.1. head dips.
There was a significant effect of sex, F (1, 66) = 6.377, p < 0.05, η p 2 = 0.088, a significant effect of diet, F (1, 66) = 7.107, p = 0.010, η p 2 = 0.097, and a significant effect of genotype, F (1, 66) = 29.143, p < 0.001, η p 2 = 0.306. Female mice made significantly more head dips than male mice ( p < 0.05) ( Figure 5 A). Mice given H. erinaceus made significantly more head dips than control mice ( p = 0.010) ( Figure 5 B) and tau mice made significantly more head dips than WT mice ( p < 0.001) ( Figure 5 C).

Head Dips in the EZM. ( A ) Female mice made significantly more head dips than male mice. ( B ) Mice given H. erinaceus made significantly more head dips than control mice. ( C ) Tau mice made significantly more head dips than WT mice. Error bars represent mean ± SEM (* p < 0.05, ** p = 0.01, *** p < 0.001).
3.5.2. Total Transitions
There was a trending effect of sex, F (1, 66) = 3.108, p = 0.083, η p 2 = 0.045. Female mice (M = 6.259, SD = 9.918) made more transitions compared to male mice (M = 3.127, SD = 4.993).
3.5.3. Percent Time Spent in the Open Arms
There was a significant effect of diet, F (1, 66) = 4.878, p < 0.05, η p 2 = 0.069, a significant effect of genotype, F (1, 66) = 10.054, p = 0.002, η p 2 = 0.132, and a significant diet × genotype interaction, F (1, 66) = 7.099, p = 0.010, η p 2 = 0.097. Mice given H. erinaceus spent significantly more time in the open arms compared to control mice ( p < 0.05). Tau mice spent significantly more time in the open arms compared to WT mice ( p < 0.01) ( Figure 6 ). Simple effects analysis revealed that tau mice given H. erinaceus spent significantly more time in the open arms compared to tau control mice ( p = 0.001) ( Figure 6 ).

Percent time spent in the open arms of the EZM. Tau mice given H. erinaceus spent significantly more time in the open arms compared to tau control mice and tau mice spent more time in the open arms compared to WT mice. Mice given H. erinaceus spent more time in the open arms compared to control mice. Error bars represent mean ± SEM (* p < 0.05, ** p < 0.01, *** p = 0.001).
3.6. Morris Water Maze
3.6.1. latency to platform.
There was a significant effect of day, F (4, 268) = 23.470, p < 0.001, η p 2 = 0.259. As the days progressed, mice found the platform faster ( Figure 7 A). A between-subjects effect of genotype was also seen, F (1, 67) = 28.018, p < 0.001, η p 2 = 0.295. Tau mice had significantly longer latencies to find the platform compared to WT mice, p < 0.001 ( Figure 7 B).

Latency to find the platform in the MWM. ( A ) As the days progressed, mice spent less time finding the platform ( p < 0.001). ( B ) Tau mice had significantly longer latencies to find the platform compared to WT mice ( p < 0.001) Error bars represent mean ± SEM.
3.6.2. Percent Time Spent in the Target Quadrant
There was a significant effect of day, F (3.444, 230.780) = 10.059, p < 0.001, η p 2 = 0.131. As the days progressed, mice spent more time in the target quadrant ( Figure 8 A). There was also a significant day × diet × genotype × sex interaction, F (3.444, 230.780) = 2.567, p < 0.05, η p 2 = 0.037. Simple effects analysis revealed that Tau female control mice spent significantly more time in the target quadrant compared to tau male control mice on day three ( p < 0.05). A between-subjects effect of genotype was also seen, F (1, 67) = 4.926, p < 0.05, η p 2 = 0.068. WT mice spent significantly more time in the target quadrant compared to tau mice ( p = 0.03) ( Figure 8 B).

Percent time spent in the target quadrant of the MWM. ( A ) As the days progressed, mice spent more time in the target quadrant ( p < 0.001). ( B ) WT mice significantly spent more time in the target quadrant compared to tau mice ( p < 0.05). Error bars represent mean ± SEM.
3.6.3. Thigmotaxicity
There was a significant effect of day, F (3.024, 202.629) = 30.430, p < 0.001, η p 2 = 0.312. As the days progressed, mice spent less time swimming along the border of the pool. A significant day × genotype interaction was also seen, F (3.024, 202.629) = 3.651, p = 0.013, η p 2 = 0.052. Simple effects analysis revealed that WT mice spent significantly less time around the border than tau mice on days 2–4 ( p < 0.001) and 5 ( p < 0.01) ( Figure 9 ). A between-subjects effect of genotype was also seen, F (1, 67) = 17.424, p < 0.001, η p 2 = 0.206. Tau mice spent significantly more time along the border than WT mice ( p < 0.001).

MWM thigmotaxicity by genotype. As the days progressed, tau mice spent significantly more time around the border of the MWM compared to WT mice. Tau mice spent significantly more time along the border on days 2–4 and 5 compared to WT mice. Error bars represent mean ± SEM. (** p < 0.01, *** p < 0.001).
3.6.4. Total Distance
There was a significant effect of day, F (4, 268) = 9.995, p < 0.001, η p 2 = 0.130. As the days progressed, mice swam shorter total distances. There was also a significant day × sex interaction, F (4, 268) = 2.724, p < 0.05, η p 2 = 0.039. While females did not show a significant decrease in total distance swam across the acquisition days, male mice did. Males swam significantly less total distance on days 3, 4, and 5 compared to day 1 ( p < 0.001) ( Figure 10 A). There was also a significant effect of genotype, F (1, 67) = 29.733, p < 0.001, η p 2 = 0.307. Tau mice traveled a significantly greater total distance than WT mice ( p < 0.001) ( Figure 10 B).

Total distance swam in the MWM. ( A ) As the days progressed, mice swam less total distance, with male mice specifically traveling shorter distances on days 3 through 5 compared to day 1 ( p < 0.001). Female mice did not show significant differences in distances throughout the training days. ( B ) As the days progressed, tau mice traveled a significantly greater distance than WT mice ( p < 0.001). Error bars represent mean ± SEM.
3.6.5. Latency to Platform (Probe Day)
There was a significant effect of genotype, F (1, 67) = 13.264, p = 0.001, η p 2 = 0.165. Tau mice had a significantly longer latency to first reach where the platform would be than WT mice ( p = 0.001) ( Figure 11 A). There was also a significant genotype × sex interaction, F (1, 67) = 7.911, p < 0.01, η p 2 = 0.106. Female WT mice took significantly less time than female tau mice to first reach where the platform would be ( p < 0.001). Additionally, female tau mice had a longer latency in first reaching where the platform would be than male tau mice ( p < 0.01) ( Figure 11 B).

MWM probe trial latency to first cross platform. ( A ) Tau mice had a significantly longer latency to first reach where the platform would be than WT mice. ( B ) Female WT mice took significantly less time than female tau mice to first cross where the platform would be and female tau mice had a significantly longer latency to first cross where the platform would be than male tau mice. Error bars represent mean ± SEM (** p < 0.01, *** p ≤ 0.001).
3.6.6. Target Crossings (Probe Day)
There was a significant effect of genotype, F (1, 67) = 7.373, p < 0.01, η p 2 = 0.099. WT mice made significantly more crosses over the target than tau mice ( p < 0.01) ( Figure 12 A). There was also a significant genotype × sex interaction, F (1, 67) = 4.533, p < 0.05, η p 2 = 0.063. Female WT mice made significantly more target crosses than female tau mice ( p < 0.01).

Additional MWM Probe Trial measures. ( A ) WT mice crossed the target significantly more than tau mice. ( B ) WT mice spent significantly more time in the target quadrant on the probe day than tau mice. ( C ) Tau mice spent significantly more time in the border (greater thigmotaxicity) than WT mice. Error bars represent mean ± SEM (* p < 0.05, ** p < 0.01).
3.6.7. Percent Time Spent in the Target Quadrant (Probe Day)
Analysis revealed a significant effect of genotype, F (1, 67) = 5.267, p < 0.05, η p 2 = 0.073. WT mice spent significantly more time in the target quadrant on the probe day than tau mice ( p < 0.05) ( Figure 12 B). There was also a trending effect of diet, F (1, 67) = 3.616, p = 0.062, η p 2 = 0.051. Mushroom mice (M = 23.054, SD = 9.64) spent less time in the target quadrant compared to control mice (M = 26.974, SD = 8.09).
3.6.8. Thigmotaxicity (Probe Day)
There was a significant effect of genotype, F (1, 67) = 10.724, p = 0.002, η p 2 = 0.138. Tau mice spent significantly more time in the border on the probe day compared to WT mice ( p < 0.01) ( Figure 12 C).
3.7. Activities of Daily Living
3.7.1. burrowing.
Analysis of pea-gravel burrowed after 2 h revealed a significant effect of genotype, F (1, 66) = 13.805, p < 0.001, η p 2 = 0.173. WT mice burrowed significantly more pea-gravel than tau mice ( p < 0.001) ( Figure 13 A). There was also a significant diet × genotype × sex interaction, F (1, 66) = 8.512, p < 0.01, η p 2 = 0.114. Female WT mushroom mice burrowed significantly more than male WT mushroom mice ( p < 0.05). Male tau mushroom mice burrowed significantly more than female tau mushroom mice ( p = 0.020). Female WT mushroom mice burrowed more than female tau mushroom mice ( p < 0.001). Male WT control mice burrowed significantly more than male tau control mice ( p = 0.020).

Burrowing assay measures. ( A ) Two-hour burrowing assessment. WT mice burrowed significantly more pea-gravel than tau mice after 2 h. ( B ) At the overnight measure, female WT mice burrowed significantly more than female tau mice. Male tau mice burrowed significantly more than female tau mice and male WT mice burrowed significantly more than male tau mice. Error bars represent mean ± SEM (* p < 0.05, *** p ≤ 0.001).
Analysis of overnight pea-gravel burrowed revealed a significant effect of genotype, F (1, 66) = 25.586, p < 0.001, η p 2 = 0.279, a significant genotype × sex interaction, F (1, 66) = 4.475, p = 0.038, η p 2 = 0.063, and a significant diet × genotype × sex interaction, F (1, 66) = 4.626, p = 0.035, η p 2 = 0.065. Male tau mice burrowed more than female tau mice ( p < 0.05) ( Figure 13 B). Female WT mice burrowed more than female tau mice ( p < 0.001). Male WT mice burrowed more than male tau mice ( p < 0.05) ( Figure 13 B). Male tau mushroom mice burrowed significantly more than female tau mushroom mice ( p < 0.001). Female tau control mice burrowed more than female tau mushroom mice ( p = 0.013). Female WT mushroom mice burrowed more than female tau mushroom mice ( p < 0.001).
3.7.2. Nesting
Nests were scored by two raters blind to experimental conditions. There was a strong agreement between the scores of both raters, α = 0.950; analysis was conducted on the resulting average nest score. There was a significant effect of genotype, F (1, 66) = 25.222, p < 0.001, η p 2 = 0.276 ( Figure 14 A). WT mice built significantly better nests than tau mice ( p < 0.001) ( Figure 14 B).

Nesting behavior. ( A ) WT mice built significantly better nests than tau mice. ( B ) Representative nest by mushroom condition and genotype; numbers represent randomly assigned IDs for raters. Error bars represent mean ± SEM (*** p < 0.001).
4. Discussion
This study sought to assess the effects of H. erinaceus on a tauopathy mouse model. We hypothesized that H. erinaceus would decrease anxiety-like behaviors, increase locomotor activity, decrease deficits in spatial memory, and improve performance in ADL measures. Overall, results indicate that H. erinaceus had significant anxiolytic effects and increased locomotor activity, in agreement with previous literature. However, H. erinaceus led to no improvements in spatial memory or activities of daily living.
Tau mice given H. erinaceus entered the center of the OFT apparatus faster than tau mice on the control diet, signaling decreased anxiety. Additionally, mice given H. erinaceus traveled a greater distance in the center of the OFT compared to control mice. These results are consistent with studies using WT mice and the OFT to assess the effects of H. erinaceus [ 30 , 31 ]. These studies showed that mice consuming H. erinaceus spent more time in the center of the OFT. Increased locomotor activity was also seen in mice consuming H. erinaceus; tau H. erinaceus mice entered the center significantly faster than tau control mice and H. erinaceus mice traveled a greater average distance in the center. This finding of increased locomotion is consistent with recent literature which showed that WT mice consuming H. erinaceus had increased locomotor activity in the Y maze [ 32 ] and longer exploration times in the emergence test, a variant assessment to the OFT [ 11 ].
Defecation is also a variable associated with emotionality, specifically stress and anxiety [ 33 , 34 , 35 ]. In the current study, males defecated significantly more than females, indicating higher levels of anxiety in males. This is a common finding consistent with past and current literature [ 33 , 36 , 37 , 38 ]. In addition to defecation denoting stress responses, it has also been suggested that males defecate more than females as a “territory marking response” [ 33 , 36 ].
In the EZM, female mice made more head dips and total transitions than male mice. This is consistent with literature that has shown that females are typically more active than males in the EZM and elevated plus maze (EPM), a similar test to the EZM used to measure anxiety-like behaviors [ 24 , 39 ]. Head dips are typically indicative of risk-taking behavior and decreased anxiety [ 40 ], meaning that in this assessment females presented increased risk-taking behaviors than males. Overall, mice given H. erinaceus spent more time in the open arms than control mice. More importantly, tau mice given H. erinaceus spent the most time in the open arms of the EZM and made more head dips than tau control mice. This is an important finding, showing that H. erinaceus has anxiolytic effects in the rTg4510 tau mouse model. A recent study [ 30 ] also indicated that WT mice supplemented with H. erinaceus spent more time in the open arms of the EPM than control mice. Additionally, it has been shown that H. erinaceus significantly increased entries into the open arm and time spent in the open arm of the EPM in WT mice [ 41 ].
Results do not reveal significant effects of H. erinaceus during the acquisition days of the MWM. However, mice did learn as the days progressed, which is consistent with literature using the MWM and transgenic mice of AD [ 42 , 43 ] and studies specifically assessing the effects of H. erinaceus on spatial memory with the MWM on AD rodent models [ 20 , 44 ]. Past literature has consistently shown spatial memory impairments in rTg4510 mice during the MWM assessment at 2.5, 3, and 5.5 months [ 21 , 45 ], and the Barnes Maze [ 46 ], with tau mice performing worse than their non-transgenic counterparts. It is also important to note that H. erinaceus has shown no effects on spatial memory in WT mice [ 32 ], which is consistent with the effects on the transgenic and non-transgenic mice used in this study. More research is warranted on assessing the effects of H. erinaceus on spatial memory in both WT and AD mouse models. Past research implying improvements on memory as an effect of the mushroom have done this with the Novel Object Recognition (NOR) task [ 11 , 15 ] which is used as a measure for short term memory rather than MWM which typically measures long term memory.
There were no significant effects of H. erinaceus on the probe day of the MWM; only significant effects of genotype were seen. This is indicative of the dietary condition having no effect on long-term memory. This contradicts previous studies assessing the effects of H. erinaceus in AD models, which have shown mice that consuming H. erinaceus performed better than mice not consuming H. erinaceus on the probe day [ 20 , 47 ]. As this is the first study assessing the effects of H. erinaceus on spatial memory in tau mice, it can help fill the gap and push further assessment of spatial memory in this mouse model by H. erinaceus supplementation.
H. erinaceus did not lead to improvements in ADL measures; only significant effects of genotype were noted. WT mice burrowed significantly more pea-gravel and built significantly better nests than tau mice. Interactions between diet, genotype, and sex were also seen. These results reveal that female WT mushroom mice burrowed significantly more than male WT mushroom mice, and that female tau mushroom mice burrowed significantly less than male tau mushroom mice in the 2-h burrowing measure. Despite no main effects of diet being seen, it is still worth reporting as not many studies assessing the effects of H. erinaceus have investigated ADL measures. Tsai-Teng et al. (2016) [ 16 ] conducted a nesting assessment with APP/PS1 mice consuming H. erinaceus . Results showed that AD mice presented more deficits in nesting activities compared to their WT counterparts; a result consistent with this study. However, in contrast with the results of the current study, researchers found that the administration of H. erinaceus was able to alleviate deficits in APP/PS1 mice in the nesting assessment [ 16 ].
Noncognitive assessments, such as ADL measures, are important to include as they can help determine non-cognitive deterioration in AD. More importantly, previous literature has shown that H. erinaceus improves non-cognitive deficits in humans consuming the mushroom in capsules 3 times a day for 49 weeks through the Instrumental Activities of Daily Living Scale (IADL) [ 5 ]. The IADL is a test of independent living skills in humans, a construct that is parallel to the ADL measure in rodent models.
Animal weights and wet food consumption increased over the course of the experiment. Tau and WT control mice weighed more than tau and WT mice given H. erinaceus . Throughout this experiment, tau and WT control mice consumed more wet food than tau and WT mice given H. erinaceus . It has been previously shown that tau mice consume more food but weigh less [ 45 ]. However, in the current study, tau mushroom mice ate less than tau control mice, indicating that not all tau mice ate more wet food. Additionally, tau female control mice weighed significantly more than WT female control mice, a finding inconsistent with previous research [ 48 ]. Female WT mice consuming H. erinaceus weighed significantly more than female tau mice consuming H. erinaceus although tau female mice ate more wet food than WT female mice. This, again, may be due to the progression of the disease in the tau mouse model as suggested by past literature [ 48 ]. Male tau mice supplemented with H. erinaceus weighed significantly more than male WT mice consuming H. erinaceus although male mice given H. erinaceus ate less throughout the experiment. Ryu et al. (2018) [ 31 ] found that the H. erinaceus did not have effects on the natural weight gain of the mice. This could be due to differences perhaps in how the diet was given; Ryu et al. (2018) [ 31 ] administered H. erinaceus by oral gavage while mice in the current study received H. erinaceus as a powder mixed into wet food. Further research is warranted on the causes of these weight differences, as a result of H. erinaceus consumption as well as sex. Additionally, when studying the effects of dietary manipulation, including food consumption and mouse weight data can be advantageous for future researchers to consider.
The hericenones and erinacines in H. erinaceus may play a role in the neurocognitive benefits seen in this mushroom [ 11 , 15 ]. More specifically, researchers have found that erinacine A is the biggest contributor to these neurological benefits out of fifteen total erinacines (A–K, P, Q, R, S) [ 5 , 19 ]. Researchers have also found that hericenones C, D, and E contribute to the synthesis of NGF, while hericenone F can reduce inflammation [ 49 ]. Thus, these hericenones and erinacines may be responsible for the anxiolytic effects seen in this study and several others [ 11 , 17 ]. Additional research into these components of H. erinaceus is certainly warranted.
There have been multiple methods of administering H. erinaceus to WT mice. Ratto et al. (2019) [ 50 ] used 21.5-month-old male WT mice. The mushroom was administered as a drink mixed with He1 mycelium and sporophore, which were ethanol extracts able to be solubilized in water for the mixture. Mice drank this ad libitum for two months. Researchers found that H. erinaceus improved recognition memory in aging mice. Ryu et al. (2018) [ 31 ] used two-month-old male WT mice and administered either 20 or 60 mg/kg of H. erinaceus powder by oral gavage for four weeks. Mice received the powder by oral gavage four times a day. It was found that the mice receiving 60 mg/kg of H. erinaceus exhibited anxiolytic and antidepressant behaviors.
There have also been multiple methods of administering H. erinaceus to transgenic mice. Mori et al. (2011) [ 15 ] used five-week-old male ICR (Institute of Cancer Research) mice with injected amyloid peptides. Fruiting bodies of H. erinaceus were turned into powder and mixed with a standard powdered diet, allowing the concentration of H. erinaceus to be 5%. The mushroom was administered to mice ad libitum for 23 days. H. erinaceus helped to ameliorate memory deficits in mice by improving recognition memory in the NOR test but did not improve exploratory behavior or locomotor activity in the Y-maze assessment. Tzeng et al. (2018) [ 20 ] used five-month-old female APP/PS1 mice, isolating H. erinaceus mycelium erinacine A and H. erinaceus mycelium erinacine S to assess the effects of each. The mushroom was administered at 10 mg/kg and 30 mg/kg a day, respectively, to experimental condition, for 100 days by oral gavage. Results indicate that erinacine A recovered cognitive impairments in spatial memory and activities of daily living (burrowing and nesting) [ 20 ]. Tsai-Teng et al. (2016) [ 16 ] also used five-month-old female APP/PS1 mice. Researchers administered 300 mg/kg per day of the mushroom for 30 days by oral gavage, isolating H. erinaceus mycelium containing erinacine A and H. erinaceus ethanol extracts. Tsai-Teng et al. (2016) [ 16 ] found that H. erinaceus mycelium recovered behavioral deficits in transgenic mice during nesting assessments.
Discussing mushroom administration in past literature is important because numerous labs and researchers may not always use the same methods, and this may perhaps be why results differ. This is the first study to administer mushroom powder mixed into wet food ad libitum to mice, with a standard rodent diet also available for the mice to feed on ad libitum . The goal was to mimic voluntary dietary supplementation in humans and eliminate stress factors which might accompany administration through techniques such as oral gavage. In each study, different quantities of H. erinaceus mycelium and respective components of the mushroom were used. All studies showing behavioral improvements, including this study, used H. erinaceus mycelium. The results of this study indicate anxiolytic effects and increased locomotor activity, but no improvements in spatial memory and noncognitive behaviors. Thus, it is important to explore the effects of different doses, erinacines, and extracts on future models of AD, including those models solely expressing tau.
One limitation in this study was the administration of the wet food. Each cage was administered 150 g of wet food for the mice to eat ad libitum . Other studies assessing the effects of H. erinaceus on WT and AD mouse models have administered the mushroom by oral gavage [ 16 , 20 , 31 ]. However, we chose to allow mice to eat ad libitum to eliminate potential stress and more closely resemble humans’ voluntary consumption of food. Due to this type of administration, we were unable to calculate the exact amount of wet food consumed by each individual mouse. Instead, the amount of wet food consumed was calculated as an average (see Section 2.4.1 ). We also collected animals’ body weights throughout the course of the experiment every 8 days to assess maturation and whether food was being consumed.
Another limitation may be the rTg4510 mouse model itself. In this specific regulatable model, the P301L tau mutation and CaMKIIa promoter system result in progressive neurofibrillary tangle pathology within the forebrain and memory deficits over time, which can be lessened when given doxycycline [ 21 , 51 ]. However, as Gamache et al. (2019) [ 52 ] report, this phenotype may not be caused solely by the expressed tau. Gamache et al. (2019) [ 52 ] report that in this model, disruptions in genes, including fibroblast growth factor 14 (Fgf14) caused by insertion of the transgene itself may be responsible for the particular phenotypes reported. While this model still allows for researchers to assess tangle pathology and behavioral deficits as a function of age, when applying an intervention aimed at alleviating behavioral or biochemical deficits, researchers must consider other factors which may be causing the deficits in the first place, beyond simply the tau mutation.
This is the first study demonstrating that H. erinaceus has anxiolytic effects in the rTg4510 tau mouse model. Previous research has shown this in relation to amyloid and WT mouse models but has not demonstrated it in a strictly tau mouse model [ 11 , 16 , 50 , 53 ]. Many other studies have assessed the cognitive effects of H. erinaceus in other models of AD or cognitive decline/aging such as APP/swePS1dE9 [ 16 , 20 ], SAMP8 [ 54 ], ICR (Institute of Cancer Research) mice with injected amyloid peptides [ 15 ], and Sprague Dawley rats injected with d-galactose [ 44 ]. This is also one of the first studies to assess the effects of H. erinaceus on both female and male mice. Previous literature assessing the effects of H. erinaceus has only used either male [ 11 , 50 , 53 ] or female mice [ 16 ], and has not assessed both sexes, with the majority of studies primarily assessing the effects on male mice.
The effects of H. erinaceus on tau mouse models have not been as consistently analyzed as those in amyloid models. Because amyloid and tau both contribute to the development of AD, it is important to study both markers and note the behavioral differences found in each model. Research has shown that tau may be a stronger underlying factor in the development of AD than Aβ [ 55 ]. By studying the effects of H. erinaceus in this rTg4510 tau mouse model, we aim to help bridge the gap between results solely seen in amyloid models; these results provide new views on differences in behavior that can be seen when using amyloid vs. tau models.
Future biochemical assessments on the effects of H. erinaceus would be informative to help understand the mechanisms by which this mushroom improves behavior. Tsai-Teng et al. (2016) [ 16 ] assessed Insulin-degrading Enzyme (IDE), NGF, Glial Fibrillary Acidic Protein (GFAP), and APP through Western Blot analysis. Results showed that H. erinaceus increased IDE levels which ameliorated Aβ plaques, increased the ratio of NGF and proNGF, decreased levels of GFAP and, interestingly, did not affect levels of APP. Future research could use immunoblots to assess levels of tau species, SOD-1, GFAP, NGF, glucocorticoid, and BDNF in this tau mouse model. Analyzing these proteins could provide information about the mushroom’s effects on tau levels, oxidation, astrocyte activity and inflammation, fiber growth and survival, and maintenance of nerve cells. As the current study demonstrated anxiolytic effects of H. erinaceus in this tau mouse model, analyzing proteins such as glucocorticoid receptors could provide a more detailed connection between the brain and behavior.
5. Conclusions
Overall, this study demonstrated that supplementation of H. erinaceus through wet food for four months has anxiolytic effects on the rTg4510 tau mouse model but leads to no improvements in spatial memory nor activities of daily living. Although no improvements were found in spatial memory, the anxiolytic effects may serve a great benefit for caretakers or those living with AD seeking to add a supplement that can help lower anxiety.
Acknowledgments
We would like to thank the Angelo State University Psychology department and the Archer College of Health & Human Services for their support. We would also like to show our gratitude to Amy Howard who was the animal welfare/lab manager throughout this project. Additional thanks go to the third cohort of graduate students in the Experimental Psychology M.S. program (Abishag Porras, Garett Parrish, Amy Howard), Jisele Olivarez, and Psychology undergraduates (Kaitlyn Huizar, Cassidy Martin, and Danielle Mullen) who volunteered their time to assist with this research. We are additionally appreciative of Kristen Craven, who read over the manuscript prior to final submission.
Funding Statement
This research received no external funding.
Author Contributions
Conceptualization, M.N.R. and S.L.P.L.; methodology, M.N.R. and S.L.P.L.; formal analysis, M.N.R.; investigation, M.N.R.; data curation, M.N.R.; writing—original draft preparation, M.N.R.; writing—review and editing, S.L.P.L.; visualization, M.N.R. and S.L.P.L.; supervision, S.L.P.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All procedures were approved by the Angelo State University Institutional Animal Care and Use Committee (IACUC) (protocol # 21-204) and were in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial
Affiliation.
- 1 Mushroom Laboratory, Hokuto Corporation, 800-8, Shimokomazawa, Nagano, 381-0008, Japan. [email protected]
- PMID: 18844328
- DOI: 10.1002/ptr.2634
A double-blind, parallel-group, placebo-controlled trial was performed on 50- to 80-year-old Japanese men and women diagnosed with mild cognitive impairment in order to examine the efficacy of oral administration of Yamabushitake (Hericium erinaceus), an edible mushroom, for improving cognitive impairment, using a cognitive function scale based on the Revised Hasegawa Dementia Scale (HDS-R). After 2 weeks of preliminary examination, 30 subjects were randomized into two 15-person groups, one of which was given Yamabushitake and the other given a placebo. The subjects of the Yamabushitake group took four 250 mg tablets containing 96% of Yamabushitake dry powder three times a day for 16 weeks. After termination of the intake, the subjects were observed for the next 4 weeks. At weeks 8, 12 and 16 of the trial, the Yamabushitake group showed significantly increased scores on the cognitive function scale compared with the placebo group. The Yamabushitake group's scores increased with the duration of intake, but at week 4 after the termination of the 16 weeks intake, the scores decreased significantly. Laboratory tests showed no adverse effect of Yamabushitake. The results obtained in this study suggest that Yamabushitake is effective in improving mild cognitive impairment.
(c) 2008 John Wiley & Sons, Ltd.
PubMed Disclaimer
Similar articles
- Ginkgo biloba: specificity of neuropsychological improvement--a selective review in search of differential effects. Kaschel R. Kaschel R. Hum Psychopharmacol. 2009 Jul;24(5):345-70. doi: 10.1002/hup.1037. Hum Psychopharmacol. 2009. PMID: 19551805 Review.
- Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, Xu Y, Murthy AK. Doody RS, et al. Neurology. 2009 May 5;72(18):1555-61. doi: 10.1212/01.wnl.0000344650.95823.03. Epub 2009 Jan 28. Neurology. 2009. PMID: 19176895 Clinical Trial.
- Chinese herbal medicine for Mild Cognitive Impairment and Age Associated Memory Impairment: a review of randomised controlled trials. May BH, Yang AW, Zhang AL, Owens MD, Bennett L, Head R, Cobiac L, Li CG, Hugel H, Story DF, Xue CC. May BH, et al. Biogerontology. 2009 Apr;10(2):109-23. doi: 10.1007/s10522-008-9163-5. Epub 2008 Aug 21. Biogerontology. 2009. PMID: 18716893 Review.
- The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. Chiu CC, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Aug 1;32(6):1538-44. doi: 10.1016/j.pnpbp.2008.05.015. Epub 2008 May 25. Prog Neuropsychopharmacol Biol Psychiatry. 2008. PMID: 18573585 Clinical Trial.
- Enhancing effect of HT008-1 on cognitive function and quality of life in cognitively declined healthy adults: a randomized, double-blind, placebo-controlled, trial. Kim J, Chung SY, Park S, Park JH, Byun S, Hwang M, Oh DS, Choi H, Kim MY, Bu YC, Doré S, Whang WW, Kim H. Kim J, et al. Pharmacol Biochem Behav. 2008 Oct;90(4):517-24. doi: 10.1016/j.pbb.2008.03.033. Pharmacol Biochem Behav. 2008. PMID: 18550156 Clinical Trial.
- Exploiting Natural Niches with Neuroprotective Properties: A Comprehensive Review. Moukham H, Lambiase A, Barone GD, Tripodi F, Coccetti P. Moukham H, et al. Nutrients. 2024 Apr 26;16(9):1298. doi: 10.3390/nu16091298. Nutrients. 2024. PMID: 38732545 Free PMC article. Review.
- Data-driven clustering approach to identify novel clusters of high cognitive impairment risk among Chinese community-dwelling elderly people with normal cognition: A national cohort study. Ran W, Yu Q. Ran W, et al. J Glob Health. 2024 Apr 19;14:04088. doi: 10.7189/jogh.14.04088. J Glob Health. 2024. PMID: 38638099 Free PMC article.
- Recent advances and role of melatonin in post-harvest quality preservation of shiitake ( Lentinula edodes ). Asdullah HU, Chen F, Hassan MA, Abbas A, Sajad S, Rafiq M, Raza MA, Tahir A, Wang D, Chen Y. Asdullah HU, et al. Front Nutr. 2024 Mar 20;11:1348235. doi: 10.3389/fnut.2024.1348235. eCollection 2024. Front Nutr. 2024. PMID: 38571753 Free PMC article. Review.
- The Relationship between Mushroom Intake and Cognitive Performance: An Epidemiological Study in the European Investigation of Cancer-Norfolk Cohort (EPIC-Norfolk). Cha S, Bell L, Williams CM. Cha S, et al. Nutrients. 2024 Jan 25;16(3):353. doi: 10.3390/nu16030353. Nutrients. 2024. PMID: 38337638 Free PMC article.
- The Acute and Chronic Effects of Lion's Mane Mushroom Supplementation on Cognitive Function, Stress and Mood in Young Adults: A Double-Blind, Parallel Groups, Pilot Study. Docherty S, Doughty FL, Smith EF. Docherty S, et al. Nutrients. 2023 Nov 20;15(22):4842. doi: 10.3390/nu15224842. Nutrients. 2023. PMID: 38004235 Free PMC article. Clinical Trial.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- The Lens - Patent Citations
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
News | African lion cub, Lomelok, euthanized at…
Share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
- Click to print (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Restaurants, Food and Drink
- Entertainment
- Immigration
- Sports Betting
News | African lion cub, Lomelok, euthanized at Lincoln Park Zoo after slow recovery from spinal surgery

Visitors brave the rain and dreary weather to see the three young cubs lounging with their father, Jabari, and other lions on heated rocks on, May 1, 2023, at Lincoln Park Zoo. The cubs turned 4 months old on May 9. (Brian Cassella/Chicago Tribune)

One of the young lion cubs bathes his brother Pilipili on May 1, 2023, at Lincoln Park Zoo. Cassella/Chicago Tribune)

Visitors brave the rain on a dreary spring day to see the lions, including three young cubs, lounging on heated rocks on May 1, 2023, at Lincoln Park Zoo. The cubs turned four months old on May 9. (Brian Cassella/Chicago Tribune)

Several of the lions, including the young cubs, watch visitors brave the rain and dreary weather on May 1, 2023, at Lincoln Park Zoo. (Brian Cassella/Chicago Tribune)

Shanna Madison / Chicago Tribune
Three lion cubs make their debut at the Pepper Family Wildlife Center on April 14, 2023, at the Lincoln Park Zoo in Chicago.

The first of three cubs gingerly enters the enclosure alongside its mother, Zari, for first time at the Pepper Family Wildlife Center on April 14, 2023, at the Lincoln Park Zoo. The cubs, all males, were born at the zoo Jan. 9 but have stayed behind the scenes, zoo officials said, with minimal human intervention. Three 3-month-old cubs named Pesho, Sidai and Lomelok will be on view to the public and exploring their habitat beginning April 15.

Lion cubs explore their new home at the Pepper Family Wildlife Center on April 14, 2023, at the Lincoln Park Zoo.

A child watches as three lion cubs are introduced to the Pepper Family Wildlife Center at the Lincoln Park Zoo.

Lion cubs explore their new home at the Pepper Family Wildlife Center at the Lincoln Park Zoo.

Jabari, the father of the three cubs, walks around the Pepper Family Wildlife Center on April 14, 2023, at the Lincoln Park Zoo.

People watch three lion cubs make their debut at the Pepper Family Wildlife Center on April 14, 2023, at the Lincoln Park Zoo in Chicago.

A lion cub explores its new home at the Pepper Family Wildlife Center.

Lomelok was born with a spinal defect that’s caused mobility challenges since he was a few weeks old, zoo officials said. He had an operation in March — the first of its kind on a lion cub — to reduce his pain and treat a herniated disc.
Lomelok’s recovery from the surgery was “slow and steady,” officials said, but he wasn’t “thriving” as he should. When veterinary staff detected a gastrointestinal obstruction that would have required another intensive surgery and a lengthy recovery, they made the “difficult but responsible decision” to euthanize Lomelok on Saturday.
“We have been overwhelmed by the support from the community for Lomelok throughout his health journey,” said Cassy Kutilek, the curator of mammals, in a Monday news release. “Lomelok’s name means ‘sweet’ in the Maa language, and that was the best way to describe him. There are no words to articulate how deeply he will be missed.”
Lomelok, who was roughly 2 pounds at birth , grew to more than 250 pounds. Zoo officials said he had started growing a thick adolescent mane. One of his favorite activities, officials said, was laying upside down and showing his white belly fur. His care team is “still processing this incredibly tough loss,” a zoo spokesperson said.
Lomelok and his two brothers, Pesho and Sidai, were born Jan. 9, 2023, at the Pepper Family Wildlife Center. His mother, Zari, gave birth after five hours of labor, earning her the title of “rock star” among zoo staff.
Lomelok’s family also included his older brother, Pilipili, his father, Jabari, and aunts Hasira and Cleo. Both of Zari’s pregnancies were part of the African Lions Species Survival Plan, a population management effort across accredited zoos within the Association of Zoos and Aquariums.
Zari “immediately attended to the cubs, started grooming them, and then, within hours of their birth, she started nursing them and feeding them,” the zoo’s curator of mammals and behavioral husbandry Mike Murray told the Tribune after the three cubs’ birth.
After some private time with little human intervention, the cubs made their public debut in April 2023, with much fanfare . Pictures and videos of Lomelok’s young life, from his birth to his first playful steps in a new home to his antics with a tree , circulated across the internet.

When Lomelok started to grow, staff were concerned about his rear limbs and lower-than-normal activity levels. He was eventually diagnosed with stenosis, which zoo officials said is the narrowing of channels that carry nerves from the spine to the legs.
“You can think of the spinal cord as a highway, carrying nerve signals from your brain out to the rest of your body,” Lomelok’s veterinarian Dr. Kate Gustavsen said in March. “Between every set of vertebrae, there are exits that the nerves leave from.”
“In Lomelok’s case, the last two exits all the way at the end of the highway have a lane closed in each direction — they’re too narrow, everybody’s irritated, the traffic is moving slowly,” she continued. “That’s causing what we see physically as his muscles not developing completely appropriately and him being clumsy in his gate, and it also causes some discomfort.”
Spinal conditions in the wild
In the wild, it’s unlikely that Lomelok would have survived very long with this condition, according to Craig Packer, a professor at the University of Minnesota and director of the Lion Research Center.
When a lion is small, its mother carries it in its mouth with its teeth around its nape. But as the cub ages, the mother would have stopped carrying it, he said. Packer estimated that Lomelok had at least an extra year of life because of his care in captivity.
“That cub would have been left behind. That cub would have either starved to death or been killed by a leopard or hyena or another lion from a neighboring pride,” Packer said. “So that animal had a much longer life than you ever would have seen in the wild.”

The challenges of surviving with this condition in the wild make it hard to know how common spinal birth defects are in lions, Packer said. Mothers keep their cubs hidden for the first few weeks of their lives, and he said some research suggests they will abandon one member of their litter.
“I’m not saying they are deliberately leaving behind disabled cubs, but I can’t say that’s impossible,” he said. “We have some evidence that they don’t always keep every single one of their cubs.”
The opportunity to see Lomelok, or any lion cub, in a zoo is a fairly rare treat because zoos have to carefully control breeding, Packer said.
“If you had a female lion who had the maximum number of surviving cubs, which would be four, and they can start breeding especially in captivity by the time they’re just a little over 2 years old, after about 37 years, the descendants would need to eat the population of Chicago every week,” Packer said.
“You can’t let them breed at their maximum, so it’s a special thing in captivity,” he added.
More in News

National News | Elon Musk drops lawsuit against ChatGPT-maker OpenAI without explanation

Health | Michigan’s largest insurer to drop weight-loss drug coverage

Politics | About half of US adults approve of Trump’s conviction, but views of him remain stable: AP-NORC poll

World News | Blinken says some of Hamas’ proposed changes to a cease-fire plan in Gaza are workable and some not
Trending nationally.
- Massachusetts middle schooler banned from wearing ‘only two genders’ shirt loses federal appeals case
- Longest-ever flight from Denver International Airport takes off Tuesday
- Oprah Winfrey hospitalized with ‘serious’ stomach issue, Gayle King says
- For Sandy Hook shooting survivors, CT high school graduation is a ‘bittersweet’ milestone
- Another COVID vaccine? Yes, and here’s why

IMAGES
VIDEO
COMMENTS
1. Introduction. Hericium erinaceus (lion's mane) is an edible mushroom, that belongs to the Hericiaceae family, order Russulales, class Agaricomycete and phylum Basidiomycota [1,2].It is extensively found in East Asian countries including Japan and China [].The mature mushroom is easily identifiable, consisting of a number of single, long, dangling fleshy spines which are white in colour [].
Neurotrophic factors are important in promoting the growth and differentiation of neurons. Nerve growth factor (NGF) is essential for the maintenance of the basal forebrain cholinergic system. Hericenones and erinacines isolated from the medicinal mushroom Hericium erinaceus can induce NGF synthesis …
Lab research shows that the anti-inflammatory effects and antioxidant properties of lion's mane may help minimize inflammation and guard your cells against damage. "Anytime we can add an anti ...
Among all culinary mushrooms, Hericium erinaceus (most commonly known as lion's mane) has been widely reported to have therapeutic activities related to the promotion of nerve and brain health. Different compounds isolated from this mushroom inducing the expression of neurotrophic factors such as nerve growth factors (NGF) have been actively ...
Researchers from The University of Queensland have discovered the active compound from an edible mushroom that boosts nerve growth and enhances memory.. Professor Frederic Meunier from the Queensland Brain Institute said the team had identified new active compounds from the mushroom, Hericium erinaceus. "Extracts from these so-called 'lion's mane' mushrooms have been used in ...
Active compounds in the edible Lion's Mane mushroom can help promote neurogenesis and enhance memory, a new study reports. Preclinical trials report the compound had a significant impact on neural growth and improved memory formation. Researchers say the compound could have clinical applications in treating and preventing neurodegenerative disorders such as Alzheimer's disease.
Background: Given the bioactive properties and limited work to date, Hericium erinaceus (Lion's mane) shows promise in improving cognitive function and mood. However, much of the human research has concentrated on chronic supplementation in cognitively compromised cohorts. Objective: The current pilot study investigated the acute and chronic (28-day) cognitive and mood-enhancing effects of ...
Background: Given the bioactive properties and limited work to date, Hericium erinaceus (Lion's mane) shows promise in improving cognitive function and mood. However, much of the human research has concentrated on chronic supplementation in cognitively compromised cohorts. Objective: The current pilot study investigated the acute and chronic (28-day) cognitive and mood-enhancing effects of ...
Hericium erinaceus is an edible and medicinal mushroom with potential neuroprotective effects. The study of H. erinaceus has attracted considerable attention during the past 10 years, particularly with regard to its potential utility in the treatment of motor dysfunction, Alzheimer disease, and othe …
Among these, the neurohealth properties of Hericium erinaceus (Bull.:Fr.) Pers., or its common names Lion's mane or Monkey's head mushroom, have been most extensively studied. Hericenones and erinacines are the two important classes of constitutes isolated from the fruiting body and mycelium of H. erinaceus , respectively ( Kawagishi et al ...
Hericium erinaceus (Lion's Mane, Stamets Host Defense, 2 grams/day): Lion's mane may produce significant improvements in cognition and function in healthy people over 50 and in MCI patients compared to placebo . Super Bifido Plus Probiotic (Flora, 1 tablet/day). ... All data from research participants described in this paper is de ...
Lion's mane mushrooms are rich in vitamins such as thiamine, riboflavin, and niacin. They are also a good source of essential minerals such as manganese, zinc, and potassium. Research suggests ...
Lion's mane extract may improve heart health, but the research to date has primarily used animal subjects. Research on rats showed that the mushroom extracts might have a cholesterol-lowering ...
Lion's mane mushroom extract may have a significant impact on the growth of brain cells and improving memory, which could inspire treatments against disorders such as Alzheimer's disease.
Lion's Mane. Lion's mane, Hericium erinaceus, is a culinary and medicinal mushroom. Lion's mane appears to have neuroprotective and antioxidant properties in the brain. Lion's Mane is most often used for Brain Health. The Examine Database covers Alzheimer's Disease,Anxiety, and 3 other conditions and goals.
Lion's mane may help ease stress, according to Best, and a 2010 study in Biomedical Research provides some evidence to support this theory. The study examines the effects of lion's mane on ...
Research to date suggests that lion's mane may help alleviate depression and anxiety. For example, a 2020 review of the literature called lion's mane "a potential alternative medicine for the treatment of depression." Likewise, a 2021 research review detailed several studies that showed significant anti-anxiety effects. Lion's mane appears to ...
Mane Research. The lion's mane has long been an iconic symbol, yet there has been no clear answer as to why lions have manes, or what function they serve. Charles Darwin was the first to suggest that the mane may be a result of sexual selection, meaning that the mane increases reproductive success. The mane may protect a male's neck during ...
Hericium erinaceus is a medicinal-culinary mushroom widely found in East Asian countries and is commonly known as lion's mane mushroom, Yamabushitake, or monkey's head mushroom . ... Recently, the present research on H. erinaceus has been focused on its antidepressant-like effects for the treatment of depressive disorder [31,32,33].
Hericium erinaceus, also known as Lion's Mane Mushroom or Hedgehog Mushroom, is an edible fungus, which has a long history of usage in traditional Chinese medicine. This mushroom is rich in some physiologically important components, especially β-glucan polysaccharides, which are responsible for anti-cancer, immuno-modulating, hypolipidemic ...
Some of these benefits include symptom improvement of sleep disorders, Alzheimer's, and Parkinson's disease. One animal study even found lion's mane to assist in neurotransmission and recognition memory. As already mentioned above, this edible fungi is also a champion for gut health. Many of the functional mushrooms, including lion's ...
Lion's mane (Hericium erinaceus) is a mushroom that grows on trunks of dead hardwood trees such as oak. It has a long history of use in East Asian medicine. Lion's mane mushroom might improve ...
Supporting cognitive function. These mushrooms contain the compounds hericenones and erinacines, which help stimulate nerve growth factor production - this contributes to brain health, memory and focus. In one study, lion's mane dietary supplements appeared to help recognition memory in mice, while another suggested the mushrooms may help ...
It noted that during the week of May 21 to 28, Google Trends showed search volumes for the phrase 'lion's mane mushroom powder' increased by 450%. And in the following week, Google searches for "benefits of lion's mane" increased by a further 120% while "what does lion's mane do" rose by 200%. Luke O'Reily, CEO at the UK-based nootropic ...
This is the first study demonstrating that H. erinaceus has anxiolytic effects in the rTg4510 tau mouse model. Previous research has shown this in relation to amyloid and WT mouse models but has not demonstrated it in a strictly tau mouse model [ 11, 16, 50, 53 ].
As more research emerges supporting the efficacy of these supplements, and as public awareness continues to grow, the adoption of NMN, NAD boosters, Lion's Mane, and LongevityPlus products is ...
A double-blind, parallel-group, placebo-controlled trial was performed on 50- to 80-year-old Japanese men and women diagnosed with mild cognitive impairment in order to examine the efficacy of oral administration of Yamabushitake (Hericium erinaceus), an edible mushroom, for improving cognitive impairment, using a cognitive function scale based on the Revised Hasegawa Dementia Scale (HDS-R).
June 7, 2024 at 5:00 a.m. A 17-month-old African lion cub at the Lincoln Park Zoo, known for his sweet and laid-back personality that captured the hearts of thousands in Chicago and on social ...