
- Plant Biology
- Human Biology
- Biology Cells Osmosis Experiment

Osmosis Experiment: Dissolving Egg Shells With Vinegar
How does osmosis keep you healthy.
Right now, as you read this, there are millions of things happening throughout your body. The food you ate just a bit ago is making its way through a watery slurry inside your stomach and small intestines. Your kidneys are working hard to excrete waste and extra water. The lacrimal glands near your eyes are secreting tears, which allow your eyelids to close without damaging your eyeballs. What’s one thing that all of these processes have in common? They all rely on osmosis: the diffusion of water from one place to another.
Osmosis factors heavily in each of these processes and is an important force for keeping every single cell in your body healthy. Osmosis is hard to see without a microscope. But if we create our very own model of a cell, using a shell-less chicken egg, we can see what happens when we manipulate the osmotic balance in the “cell”!

- 3 glasses (large enough to fit the egg plus liquid)
- 3 butter knives
- White vinegar (about 3 cups)
- Distilled water (about 2 cups)
- Light corn syrup (about 1 ¼ cups)
- Slotted spoon
- Measuring cup (1 cup)
- Measuring spoons (1 tablespoon and ½ tablespoon)
- Sticky notes and marker
- Scale (optional)
Note : It’s okay to touch the eggs, but remember to wash your hands afterwards to avoid any nasty surprises!
1. Place one egg in each glass. Pour in enough vinegar to cover each egg. Bubbles will start to form around the egg, and it’ll float up. To keep it submerged, put a butter knife in the glass to hold it down.
2. Put the three glasses in the refrigerator and allow to sit for 24 hours.
3. Gently holding the egg in the glass, pour out the old vinegar. Replace with fresh vinegar, and let sit in the refrigerator for another 24 hours. Repeat this process until the shells are fully dissolved and only the membrane remains. This should take about 2-3 days.
4. Gently remove the eggs using the slotted spoon and rinse with tap water in the sink. Rinse out the empty glasses as well.
5. Gently put the shell-less eggs aside for a moment on a plate.
6. Prepare three different sugar-water solutions as follows, labeling with sticky notes:
Glass 1: Label “hypertonic”. Pour in one cup of corn syrup.
Glass 2: Label “isotonic”. Add 1 ½ tablespoons corn syrup to the one cup measuring cup, and fill the remainder with distilled water. Pour into glass (make sure you get all the corn syrup out!) and stir to dissolve.
Glass 3: Label “hypotonic”. Pour in one cup of distilled water. Gently put one shell-less egg in each of the glasses, and let sit in the refrigerator for another 24 hours.
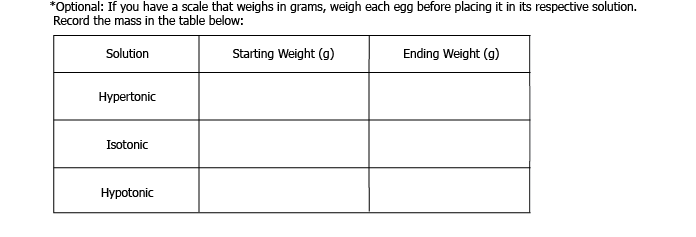
7. Remove the glasses from the refrigerator, and gently put the eggs on a plate. If you weighed the eggs before putting them in each solution, weigh them again. What happened to each of the eggs?

How does osmosis work?
Osmosis is the scientific term that describes how water flows to different places depending on certain conditions. In this case, water moves around to different areas based on a concentration gradient , i.e. solutions which have different concentrations of dissolved particles ( solutes ) in them. Water always flows to the area with the most dissolved solutes, so that in the end both solutions have an equal concentration of solutes. Think about if you added a drop of food dye to a cup of water – even if you didn’t stir it, it would eventually dissolve on its own into the water.
In biological systems, the different solutions are usually separated by a semipermeable membrane , like cell membranes or kidney tubules . These act sort of like a net that keeps solutes trapped, but they still allow water to pass through freely. In this way, cells can keep all of their “guts” contained but still exchange water.
Now, think about the inside of an egg. There’s a lot of water inside of the egg, but a lot of other things (i.e. solutes) too, like protein and fat. When you placed the egg in the three solutions, how do you think the concentration of solutes differed between the inside of the egg and outside of the egg? The egg membrane acts as a semipermeable membrane and keeps all of the dissolved solutes separated but allows the water to pass through.
How did osmosis make the eggs change size (or not)?
If the steps above work out properly, the results should be as follows.
In the case of the hypertonic solution, there were more solutes in the corn syrup than there were in the egg. So, water flowed out of the egg and into the corn syrup, and as a result the egg shriveled up.
In the case of the isotonic solution, there was roughly an equal amount of solutes in the corn syrup/water solution than there was in the egg, so there was no net movement in or out of the egg. It stayed the same size.
In the case of the hypotonic solution, there were more solutes in the egg than in the pure water. So, water flowed into the egg, and as a result, it grew in size.

Osmosis and You
Every cell in your body needs the right amount of water inside of it to keep its shape, produce energy, get rid of wastes, and other functions that keep you healthy.
This is why medicines that are injected into patients need to be carefully designed so that the solution has the same concentration of solutes as their cells (i.e. isotonic). If you were sick and became dehydrated, for example, you would get a 0.90% saline IV drip. If it were too far off from this mark it wouldn’t be isotonic anymore, and your blood cells might shrivel up or even explode , depending on the concentration of dissolved solutes in the water.
Osmosis works just the same way in your cells as it does in our egg “cell” model. Thankfully, though, the semipermeable membrane of the egg is much stronger, so you don’t have to worry about the egg exploding as well!
Related Topics
Choose one of the following categories to see related pages:.
- Experiments
Share this Page
Lindsay graduated with a master’s degree in wildlife biology and conservation from the University of Alaska Fairbanks. She also spent her time in Alaska racing sled dogs, and studying caribou and how well they are able to digest nutrients from their foods. Now, she enjoys sampling fine craft beers in Fort Collins, Colorado, knitting, and helping to inspire people to learn more about wildlife, nature, and science in general.
- Basic Types of Cells
- Cell Organelles
- Connective Tissue Cells
- Epithelial Cells
- Introduction to Cells
- Muscle Cells
- Nerve Cells
- Osmosis Experiment
- Structure of the Cell Nucleus
- Structures of the Cell Cytoplasm
Science Newsletter:
Full list of our videos.

Teaching Biology?

How to Make Science Films

Read our Wildlife Guide

New From Untamed Science


BIOLOGY JUNCTION
Test And Quizzes for Biology, Pre-AP, Or AP Biology For Teachers And Students
Osmosis & Diffusion in Egg Lab
Objective: In this investigation, you will use a fresh hen’s egg to determine what happens during osmosis & diffusion across membranes.
Materials: (per lab group) 1-2 fresh hen eggs in their shells, masking tape & marker, distilled water, clear sugar syrup (Karo, for example), vinegar, clear jar with lid, tongs, electronic balance, paper towels, paper, pencil
- Label the jar with your lab group & the word “vinegar”.
- Mass the egg with the electronic balance & record in the data table.
- Carefully place the raw egg into the jar & cover the egg with vinegar.
- Loosely re-cap the jar & allow the jar to sit for 24 to 48 hours until the outer calcium shell is removed.
- Open the jar & pour off the vinegar.
- Use tongs to carefully remove the egg to a paper towel & pat it dry.
- Record the size & appearance of your egg in your data table.
- Mass the egg on an electronic balance & record.
- Clean and re-label the jar with your lab group & the word “distilled water”.
- Carefully place the egg into the jar & cover the egg with distilled water.
- Loosely re-cap the jar & allow it to sit for 24 hours.
- Open the jar & discard the distilled water.
- Clean and re-label the jar with your lab group & the word “syrup”.
- Carefully place the egg into the jar & cover the egg with clear syrup.
- Open the jar & pour off the syrup.
- Use tongs to very carefully remove the egg & rinse off the excess syrup under slow running water.
- Pat the egg dry on a paper towel.
- Clean up your work area & put away all lab equipment.
Questions & Conclusion:
1. Vinegar is made of acetic acid & water. Explain how it was able to remove the calcium shell.
2. (a) What happened to the size of the egg after remaining in vinegar?
(b) Was there more or less liquid left in the jar?
(c) Did water move into or out of the egg? Why?
3. (a) What happened to the size of the egg after remaining in distilled water?
4. (a) What happened to the size of the egg after remaining in syrup?
5. Was the egg larger after remaining in water or vinegar? Why?
6. Why are fresh vegetables sprinkled with water at markets?
7. Roads are sometimes salted to melt ice. What does this salting do to the plants along roadsides & why?
Egg Osmosis Experiments With Distilled Water & Salt Water

Osmosis happens when a solvent, like distilled water, diffuses across a membrane into a solution that has a higher concentration of some solute, like salt water. Eggs are a model system for demonstrating osmosis because the thin membrane that lies underneath the shell is permeable to water, providing a system that changes volume as water passes in or out of the egg's interior.
Goal of the Experiment
Inside the egg membrane is a concentrated solution of proteins and water. When the egg is soaked in distilled water, osmosis causes water to diffuse into the egg to equalize the concentration of water on both sides of the membrane, and the egg increases in volume. If that same egg is then soaked in concentrated salt water, osmosis causes the water to diffuse back out of the egg, and the egg decreases in volume. The goal of the experiment is to demonstrate the process of osmosis by measuring the change in volume of the egg and then relate this to how water moves in and out of living cells.
Time Requirements
If only one experiment is performed on each individual egg, you will need to plan on three days for the experiment. Two days may be required to dissolve the egg shell with vinegar so that only the rubbery membrane remains. One day is required to complete each osmosis experiment on a single egg. Demonstrating osmosis in both directions, diffusion of water into the egg and then out of the egg, will require an additional 24 hours, for a total of four days.

Material Requirements
In addition to the eggs and vinegar to dissolve the shell, you will need plastic cups or glassware to store the eggs while soaking, salt to make a concentrated salt solution, and some way to measure the change in volume of the egg, such as rulers to measure the egg's dimensions, balances to measure the change in mass, or graduated glassware to measure displaced volume. Keep a stock of cleaning supplies nearby to deal with broken eggs.
Experimental Variations
Simple variations can be made to the experiment to make it more interesting. Food coloring can be added to the distilled water to demonstrate with color that water from the cup is moving inside the egg. After the egg swells in size, it can be popped and colored water will come out. Solutions other than salt water can also be used to cause water to diffuse out of the egg, such as oils or syrups that have little to no water content. These will cause a larger decrease in the egg's volume than salt water.
- Oregon State University, Agriculture in the Classroom: Egg Science: Dissolution & Osmosis
- ILoveBacteria.com: Egg Osmosis
- Penn State Materials Research Institute, Education and Outreach Programs: Osmosis Eggs
- CSIRO: Easter Egg Eggs-periments for Kids
- Fermilab ARISE Project: Osmosis Egg Lab
Cite This Article
Bush, Joshua. "Egg Osmosis Experiments With Distilled Water & Salt Water" sciencing.com , https://www.sciencing.com/egg-osmosis-experiments-distilled-water-salt-water-11910/. 13 March 2018.
Bush, Joshua. (2018, March 13). Egg Osmosis Experiments With Distilled Water & Salt Water. sciencing.com . Retrieved from https://www.sciencing.com/egg-osmosis-experiments-distilled-water-salt-water-11910/
Bush, Joshua. Egg Osmosis Experiments With Distilled Water & Salt Water last modified March 24, 2022. https://www.sciencing.com/egg-osmosis-experiments-distilled-water-salt-water-11910/
Recommended

Osmosis Eggsperiment
Introduction.
Water passes into and out of cells by a special type of diffusion called osmosis . Osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher water concentration to an area of lower water concentration. All of our cells are surrounded by a selectively permeable membrane through which water molecules can pass. In this simple experiment, your students will use an egg membrane to model how osmosis works in animal cells.
Next Generation Science Standards
- LS1.A: Structure and Function. Within cells, special structures are responsible for particular functions, and the cell membrane forms the boundary that controls what enters and leaves the cell.
- Science and Engineering Practices: Developing and Using Models
- Crosscutting Concepts: Structure and Function
Considerations
This activity works best for students working in groups of 2 to 3 and takes place over 3 days as follows:
- Day 1: Dissolving Eggshells 15minutes
- Day 2: Setting up the experiment 30minutes
- Day 3: Recording data and completing a lab report 45minutes

Per Student Group:
- 2 Fresh Eggs
- White Vinegar (about 600 mL)
- 2 Containers (large enough to hold an egg and completely cover it with liquid; 600-mL beakers work well)
- Large Spoon
- Distilled Water (about 300 mL)
- Corn Syrup (about 300 mL)
- 2 Small Paper Plates
- Grease Pencil
Preparation and Procedure
The first step is to dissolve the eggshell and expose the membrane. To do this, students soak the eggs in vinegar for 24 hours. Vinegar contains acetic acid that reacts with the calcium carbonate in the shell. When students first place the eggs in vinegar, have them observe the tiny bubbles forming around the eggs. This is evidence that a chemical reaction is taking place. The procedures below include the steps for dissolving the shells and completing the experiment.
- Use the grease pencil to label one container and one paper plate “Egg 1” and the other container and paper plate “Egg 2.” Carefully place an egg into each container.
- Pour enough vinegar over each egg to completely cover it.
- Observe the eggs for a few minutes and note any changes.
- Leave the eggs in their containers for 24 hours.
- Observe the eggs the next day and record your observations.
- Slowly pour the vinegar out of each container. Be very careful not to rupture the egg membranes.
- Carefully remove the eggs using the tablespoon, rinse them with water, and place each on its own labeled paper plate. Set the containers aside for now.
- Measure and record the mass of each egg, then place each egg back into its original container.
- Pour distilled water into the Egg 1 container until the egg is completely covered.
- Pour corn syrup into the Egg 2 container until the egg is completely covered.
- Put the 2 containers in a safe place overnight. Note: Have students make a prediction about what they think will happen to the mass of each egg .
- After 24 hours, observe each egg and record your observations.
- Slowly pour the water or syrup out of each container. Be very careful not to rupture the egg membranes.
- Carefully remove the eggs using the spoon, rinse them with water, and place each on its own labeled paper plate.
- Measure and record the mass of each egg. Calculate and record the change in mass.
Sample Data Table
Students should observe that the egg in distilled water was plump and gained mass, while the egg that was in corn syrup was shriveled and lost mass.
After the experiment, share with your students that egg white is about 90% water and discuss with them how the egg membrane (like a cell membrane) is selectively permeable. It lets some molecules move through—such as water, while it blocks larger molecules—such as sugar.
From students’ understanding of osmosis and diffusion, they should be able to explain that placing the egg in distilled water caused water to move from outside of the egg, where the concentration was higher, to inside of the egg, where the concentration was lower. The reverse happened for the egg placed in corn syrup. Because corn syrup contains a high amount of sugar, water molecules moved from the inside of the egg to an area of lower concentration outside of the egg.
- Have students think of a way to make the shriveled egg plump again.
- Have students plan and conduct investigations using other solutions such as salty water, and also with food coloring.
- Have students create a drawing showing how osmosis works. They may also create a physical model using candy pieces to represent water molecules.
Shop the Kit

Additional Reference Kits
- Carolina® Osmosis Chamber Set #684270
Explore More Activities
Leave your print: basic fingerprinting, simulating macroinvertebrate sampling for assessing freshwater quality, energize claims and evidence through smithsonian science for the classroom, the fusion of science and language through smithsonian science for the classroom, unlocking science success: navigating middle school learning progressions, get free activities to your inbox.
- Author Profile
- Posts by the Author
- Webinar: How High-Quality Science Programs Can Improve Reading and Math Scores
- Tips for Cleaning Microscopes
- Teacher’s Guide to Microscopes
- How to Use a Student Compound Microscope
- The Case of The Murdered Mayor – Solve a Forensic Case Using Multiple Lines of Evidence
- Modeling DNA to Protein: Go Hands on with Protein Synthesis and Mutation

Carolina Staff
Carolina is teamed with teachers and continually provides valuable resources–articles, activities, and how-to videos–to help teachers in their classroom.
Hands-On with Photosynthesis and Cellular Respiration
Diffusion—molecules on the move, you may also like, modeling dna to protein: go hands on with..., featured creatures, photosynthesis and cellular respiration: teaching common biology concepts..., journey through the heart, next generation dissection: form, function, and frogs, what goes on inside a spectrophotometer, energize claims and evidence through smithsonian science for..., the fusion of science and language through smithsonian..., leave a comment cancel reply.
Save my name, email, and website in this browser for the next time I comment.
Subscribe to Newsletter
Sign up for free resources delivered to your inbox!
- Biology Topics
- Dissection Resources
- Advanced Placement
- Carolina Correlations
- Carolina 3D
- Video Library
Get Started
- School Year Planning
- Buying Guides
- Carolina Essentials
- Media Center
- Privacy & Security
- 800.334.5551
- [email protected]
- 2700 York Rd, Burlington, NC 27215-3398

This website uses cookies to improve your experience. We'll assume you're ok with this, but you can opt-out if you wish. Accept Read More
Remember Me
Forgot Password?

IMAGES
VIDEO
COMMENTS
Osmosis Experiment: Dissolving Egg Shells With Vinegar. ... They all rely on osmosis: the diffusion of water from one place to another. Osmosis factors heavily in each of these processes and is an important force for keeping every single cell in your body healthy. Osmosis is hard to see without a microscope.
Having each small group design an experiment with one egg will allow you to do the activity with fewer eggs per class, and collecting several sets of data will enable students to identify any outliers. This Snack is an excellent activity for introducing diffusion, osmosis, and the semipermeability of membranes and allows learners to engage in ...
Diffusion is the spontaneous movement of any substance spreading from a higher concentration to a lower concentration, ... Osmosis is similar, but is particular to solutions (dissolved mixtures) separated by a membrane. Osmosis is the process in which water moves through a membrane. ... Prior Experiment - make a Naked Egg.
Osmosis & Diffusion in an Egg Objective: In this investigation, you will use a fresh hen's egg to determine what happens during osmosis & diffusion across membranes. Materials: (per lab group) 1-2 fresh hen eggs in their shells, masking tape & marker, distilled water, clear sugar syrup (Karo,…
THE INCREDIBLE EGG . How to Have Fun with Diffusion and Osmosis . Demo or Lab . To Prepare Decalcified Eggs: • Submerge raw eggs in vinegar in a container for approximately 24-30 hours. (plastic and metal containers not good) • Use plenty of vinegar and at the end of the soaking time; gently scrape off remaining
Try this simple experiment in order to see diffusion and osmosis work with an egg. This experiment helps demonstrate how a cell moves objects into and out of...
Two days may be required to dissolve the egg shell with vinegar so that only the rubbery membrane remains. One day is required to complete each osmosis experiment on a single egg. Demonstrating osmosis in both directions, diffusion of water into the egg and then out of the egg, will require an additional 24 hours, for a total of four days.
Water passes into and out of cells by a special type of diffusion called osmosis. Osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher water concentration to an area of lower water concentration. ... After the experiment, share with your students that egg white is about 90% water and discuss ...
2. Use an interactive white board to show the animations of diffusion and osmosis (links are located on the bottom of the diffusion and osmosis pages). 3. Read over the first page of the egg osmosis lab with the students and guide them in filling out the pre-lab and hypothesis questions. o Techniques and Activities Assign students to groups of ...
Abstract. This laboratory experiment explores the principles of diffusion and osmosis using eggs as semi-permeable membranes. The goal is to understand how changes in the external environment affect the internal environment of the egg.