Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF

Case Study on Acute Gastroenteritis

Gastroenteritis, sometimes referred to as “stomach flu”, is an inflammation of the GI (gastrointestinal) tract, which includes the stomach and intestines. Most cases of gastroenteritis are caused by viruses. Bacterial gastroenteritis (caused by bacteria) often causes severe symptoms. It can even be fatal. It is also the most common digestive disorder among children. Severe gastroenteritis causes dehydration and an imbalance of blood chemicals (electrolytes) because of a loss of body fluids in the vomit and stool. This can be acquired through contaminated food and water that contains harmful bacteria (such as salmonella, Campylobacter, and E. coli). Food can be contaminated when food handlers don’t wash their hands. Or when food isn’t stored, handled, or cooked correctly. This can also be acquired and spread through the fecal-oral route, people with gastroenteritis have harmful bacteria in their stool. When they don’t wash their hands well after using the bathroom, they can spread the germs to objects. If you touch the same objects, you can pick up the germs on your hands and transfer them to your mouth. (Fairview.org, 2021)
Related papers
Journal of Experimental Medicine, 1953
Feeding of stool supernates to volunteers has demonstrated that there are at least two different types—afebrile and febrile—of acute infectious non-bacterial gastroenteritis. The afebrile illness, produced by the Marcy agent previously described, has an average incubation period of 60 hours and is characterized by watery diarrhea. The agent (FS) responsible for the febrile illness has been carried through 3 human passages. The febrile disease has an average incubation period of 27 hours and is characterized by constitutional symptoms. It is believed that the two agents are not the same because of the differences in incubation period, the clinical picture, and the absence of cross-immunity. Intranasal instillation of throat washings obtained from 2 persons with gastroenteritis failed to produce illness in 2 groups of volunteers.
Emerging Infectious Diseases, 2010
Journal of Infectious Diseases, 2012
Current Treatment Options in Gastroenterology, 1999
ACUTE GASTROENTERITIS IN CHILDREN. CURRENT EPIDEMIOLOGICAL AND ETIOLOGICAL CONSIDERATIONS (Abstract): Acute diarrheal disease ranks second among the causes of specific morbidity (after respiratory infections) with higher incidence values in economically undeveloped or developing countries (3 to 7 episodes of diarrhea / year / child compared to only 1-2 episodes / year / child in highly industrialized countries). Infection with Campylobacter globally was the most frequently reported zooanthroponosis, with 214,268 confirmed human cases in 2012. Almost any pathogen involved in the etiology of acute diarrheal disease may be involved in the occurrence of healthcare-associated infections; among these, the rotavirus and Clostridium difficile are most prevalent. General hygiene measures change the epidemiological aspect of acute diarrheal disease by modifying the factors which favor the transmission of pathogens.
European Journal of Clinical Microbiology & Infectious Diseases, 2013
Academia Letters, 2021
Dedico este libro a los hombres y mujeres del Grupo INJOY, todos los cuales están comprometidos absolutamente con la misión de ayudar a otros a hacer de los fracasos algo positivo. Betania es un sagello de Editorial Caribe
KÜRESELLEŞME SÜRECİNDE DEVLETLERARASI İLİŞKİLER SİYASAL İLETİŞİM VE KAMU DİPLOMASİSİ, 2019
JAAL Volume 12, Number 2, 2023
UNESCO, 2024
DergiPark (Istanbul University), 2024
arXiv: General Physics, 2010
Borobudur, Java and Southeast Asia in the Late Eighth Century, 2023
Journal of Affective Disorders, 2011
Micromachines
Clinical Kidney Journal, 2014
Cooperation and Conflict, 2017
Manuscripta Mathematica, 1991
IEEE Antennas and Propagation Magazine, 2019
Proceedings of The Royal Society B: Biological Sciences, 2009
Adam Somorjai - Bernard Sawicki, 2015
Journal of Adolescent Research, 2006
ChemistrySelect, 2020
Related topics
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Case Study: Salmonella Gastroenteritis in a 4-Month Old Infant
Alec A. Rudentein, MS3 Rowan SOM; Raveena K. Midha, MS3 Rowan SOM; Puthenmadam Radhakrishnan MD, MPH, FAAP
INTRODUCTION
Salmonella gastroenteritis is an infection that can result in serious and life-threatening complications in the pediatric population. Infants below the age of 12 months are especially at an increased risk of morbidity and mortality. Our case is a 4-month-old male who presents with gastroenteritis in the ED and evaluated for sepsis. Stool cultures were taken and resulted in a positive salmonella gastroenteritis diagnosis. Gastroenteritis is a common presentation in infants and is often not infectious in etiology.We present this case because it is imperative to acknowledge that salmonella infection is a potential and serious cause of gastroenteritis in infants. A search of literature resulted in many mentions of statistics and epidemiology; there are very scant case reports on Salmonella infections in infants.
CASE DESCRIPTION
This is a case of a 4-month-old male with no significant past medical history, born at full term who presented to the emergency department with reports of a fever and loose stools. Patient’s mother reported that onset of symptoms was 6 hours prior to presentation and temperature at home was 102.2 F.The patient had no sick contacts; no recent travel, no pets at home and all immunizations were up to date. Initial VS were significant for a rectal temperature of 105.9 and a HR of 200 BPM. Physical exam showed yellowish stools with streaks of blood, all other findings were unremarkable. Laboratory findings were significant for an elevated absolute neutrophil count (7.38×10^3/mcl), an elevated absolute lymphocyte count (0.72 x 10^3/mcl), and an elevated CRP (2.0 mg/dL). Urinalysis was normal and the patient was negative for influenza, COVID-19, and RSV. XR of chest/ abdomen showed no acute abnormalities. Patient was admitted to the pediatric in-patient floor for further evaluation and a stool culture was ordered.
On the pediatric floor, the patient was given acetaminophen and IV fluid for hydration. A sepsis evaluation including lumbar puncture with CSF cultures and blood cultures were performed on hospital day 2. Stool culture obtained were positive for Salmonella species. After a discussion with pediatric ID, it was decided that the patient would be placed on IV ceftriaxone. The patient’s diarrhea began decreasing on hospital day 5 and stools remained non-bloody for more than 24 hours. Blood culture and CSF cultures showed no growth after 2 days. Patient’s intake increased to 4 oz of fullstrength formula every 4 hrs. After the patient was afebrile for approximately 36 hours, he was discharged on hospital day 6 and placed on azithromycin PO for a duration of 5 days.
Salmonella is a motile, gram-negative facultative anaerobic bacilli as part of the Enterobacteriaceae family.There are numerous Salmonella serotypes and species; however, this case focuses on non-typhoidal Salmonella species and their particular deleterious effects in infants. Most non-typhoidal Salmonella infections are acquired through food-borne contaminants. Frequent transmission of non-typhoid Salmonella infections occurs due to the consumption of contaminated animal-based food, such as eggs, meat, dairy products, contaminated water, or poor hygiene [3]. The infections can be self-limiting or progress to more advanced states. In addition, formula is also a potential nidus for Salmonella infection. The improper storage of formula is the most likely cause of formula-caused Salmonella infection [3]. Furthermore, Salmonella , unlike many other enteric pathogens, have an asymptomatic carrier state which can help spread the disease. A common way for the pathogen to spread to newborns and children is through maternal asymptomatic carriers [1,3].
In the case presented, it was thought that the patient was exposed to Salmonella via mishandling of poultry or an asymptomatic carrier.As there was no local outbreak of Salmonella from the formula, the Department of Health decided not to pursue the formula avenue. Likewise, the patient had no recent travel, no sick contacts and no pets at home.The patient’s mother cooks meals for the family so it can be speculated that the pathogen spread from the food to the mother to the patient.
Salmonella causes its effects locally within the gastrointestinal site as well as distantly through its ability to invade the intestinal mucosa and replicate within the lamina propria. From there, it can invade the mesenteric lymph nodes and spread to the rest of the body. Salmonella gastroenteritis is commonly associated with diarrhea, first starting with watery diarrhea and possibly progressing to bloody or mucus-containing diarrhea due to its invasive properties [1,3]. In immunocompetent adults, Salmonella gastroenteritis is eliminated through the body’s immune response, the naturally occurring enteric flora, gastric acid, and motility, as well as the intestinal mucus. Each aspect works to remove the pathogen as well as form protective barriers to prevent the organism from acclimating to the host’s internal environment. Infants and children lack or have an immature defense system and are thus at increased risk of developing more serious complications of Salmonella infections [1,4].
Noting Salmonella as the cause of gastroenteritis is imperative due to the systemic effects the organism can have. “Bacteremia may occur in 30-50% of neonates infected with Salmonella , including those with no evidence of gastroenteritis. Focal infections of almost every organ system (for example bone, joint, lung) are reported with Salmonella gastroenteritis, but meningitis is the most feared of these complications and emphasizes the vigilance required to evaluate infants who are infected with Salmonella ” [1].The peak incidence of Salmonella bacteremia and meningitis occur in infants less than 2 months of age [2]. According to the CDC, infants, especially those who are not breastfed or have a weakened immune system, are more likely to get an invasive Salmonella infection and should be treated with antibiotics [3,4].
It is recommended that the use of antibiotics be limited in immunocompetent individuals aged 12 months to 50 years old with acute salmonella gastroenteritis because of the self-limiting course of disease. It is established that patient’s less than 3 months of age are given antibiotics due to a risk of complications such as sepsis and meningitis [9]. However, there is little evidence to support that antibiotics should be given from 3 months to 12 months of age. According to a review of literature in 2017, the current recommended guidelines for a patient between 3 months-12 months of age is no treatment required if the patient appears well and non-toxic. If the patient is unwell or toxic appearing, then blood culture, with or without CSF culture, should be obtained and parenteral antibiotics should be started. If the blood culture shows no growth at 48 hours and the patient appears well, then the patient can be switched to oral antibiotics [6]. Antibiotic therapy duration for immunocompetent children is recommended at 3-14 days [5]. According to these guidelines, it was necessary to place our patient on antibiotic therapy due to multiple bouts of bloody diarrhea and persistently high fever, dehydration and general ill appearance.
The mainstay treatment of Salmonella gastroenteritis for adults and adolescents is an oral dose of fluoroquinolones because of their antimicrobial activity against gram-negative enteric pathogens. Some data has shown that fluoroquinolones could potentially be safe during short courses of antibiotics for children. However, previous data on animal models suggests that fluoroquinolones can cause joint toxicity and cartilage damage. As a result, they are not typically prescribed in children [8]. Alternatives to fluoroquinolones are other oral antibiotics such as TMP-SMX, cefixime and azithromycin. Due to this patient’s poor oral intake and peripheral IV access, IV ceftriaxone was a reasonable choice of antimicrobial therapy. Ceftriaxone has been shown to be as effective as oral ciprofloxacin in children with acute invasive diarrheas [ 7]. In addition, ceftriaxone eliminates the risk of joint toxicity in vulnerable pediatric patients such as this 4-month-old male. In this case, the duration of parenteral antibiotic therapy was 3 days. Following discharge, the patient was prescribed azithromycin for 5 additional days for a total of 7 days which falls in the recommended guidelines discussed above.
Gastroenteritis in infants, particularly children under 12 months of age, is a common condition with a range of causes. In many cases, the illness is self-limited and no antibiotics or treatments are needed. However, it is important to note that Salmonella is also a common cause of gastroenteritis and should always be included in the differential diagnosis. In the case presented, the patient was brought to the ED for a sepsis workup and it was most likely due to the presentation of bloody stools that the stool culture was ordered. However, Salmonella’s presentation can vary from watery stools to asymptomatic infections.Though the clinical presentations differ, Salmonella’s potential systematic effects can prove to be fatal. Therefore, Salmonella gastroenteritis should be considered in the differential diagnosis due to its ability to invade the lymphatic system and spread to cause more systemic infections.
- Kinney JS, Eiden JJ. Enteric infectious disease in neonates. Epidemiology, pathogenesis, and a practical approach to evaluation and therapy. Clin Perinatol. 1994 Jun;21(2):317- 33. doi: 10.1016/S0095-5108(18)30348-8. PMID: 8070229; PMCID: PMC7133246.
- Nelson SJ, Granoff D. Salmonella gastroenteritis in the first three months of life. A review of management and complications. Clin Pediatr (Phila). 1982 Dec;21(12):709-12. doi: 10.1177/000992288202101201. PMID: 7140121.
- Bula-Rudas FJ, Rathore MH, Maraqa NF. Salmonella Infections in Childhood. Adv Pediatr. 2015 Aug;62(1):29-58. doi: 10.1016/j.yapd.2015.04.005. PMID: 26205108.
- “Questions and Answers.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 5 Dec. 2019, www.cdc.gov/salmonella/general/index.html.
- Sirinavin, S, and P Garner. “Antibiotics for Treating Salmonella Gut Infections.” Cochrane database of systematic reviews 2 (2000): CD001167–CD001167. Print.
- Wen, Sophie CH, Emma Best, and Clare Nourse. “Non- Typhoidal Salmonella Infections in Children: Review of Literature and Recommendations for Management: Non- Typhoidal Salmonella Infections.” Journal of paediatrics and child health 53.10 (2017): 936–941.Web.
- LEIBOVITZ, EUGENE et al. “Oral Ciprofloxacin Vs. Intramuscular Ceftriaxone as Empiric Treatment of Acute Invasive Diarrhea in Children.” The Pediatric infectious disease journal 19.11 (2000): 1060–1067.Web.
- GRADY, RICHARD. “Safety Profile of Quinolone Antibiotics in the Pediatric Population.” The Pediatric infectious disease journal 22.12 (2003): 1128–1132.Web.
- ST. GEME, JOSEPH W et al. “Consensus: Management of Salmonella Infection in the FirstYear of Life.”The Pediatric infectious disease journal 7.9 (1988): 615–621.Web.
Share This Story, Choose Your Platform!
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Acute gastroenteritis in primary care: a longitudinal study in the Swiss Sentinel Surveillance Network, Sentinella
Claudia schmutz, philipp justus bless, daniel mäusezahl, marianne jost, mirjam mäusezahl-feuz.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Contributed equally.
Received 2017 May 16; Accepted 2017 Jul 19; Issue date 2017.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Acute gastroenteritis (AG) leads to considerable burden of disease, health care costs and socio-economic impact worldwide. We assessed the frequency of medical consultations and work absenteeism due to AG at primary care level, and physicians’ case management using the Swiss Sentinel Surveillance Network “Sentinella”.
During the 1-year, longitudinal study in 2014, 172 physicians participating in “Sentinella” reported consultations due to AG including information on clinical presentation, stool diagnostics, treatment, and work absenteeism.
An incidence of 2146 first consultations due to AG at primary care level per 100,000 inhabitants in Switzerland was calculated for 2014 based on reported 3.9 thousand cases. Physicians classified patients’ general condition at first consultation with a median score of 7 (1 = poor, 10 = good). The majority (92%) of patients received dietary recommendations and/or medical prescriptions; antibiotics were prescribed in 8.5%. Stool testing was initiated in 12.3% of cases; more frequently in patients reporting recent travel. Among employees (15–64 years), 86.3% were on sick leave. Median duration of sick leave was 4 days.
Conclusions
The burden of AG in primary care is high and comparable with that of influenza-like illness (ILI) in Switzerland. Work absenteeism is substantial, leading to considerable socio-economic impact. Mandatory infectious disease surveillance underestimates the burden of AG considering that stool testing is not conducted routinely. While a national strategy to reduce the burden of ILI exists, similar comprehensive prevention efforts should be considered for AG.
Electronic supplementary material
The online version of this article (doi:10.1007/s15010-017-1049-5) contains supplementary material, which is available to authorized users.
Keywords: Acute gastroenteritis, Sentinel surveillance, Primary health care, Switzerland, Antibiotics, Infectious intestinal diseases
Acute gastroenteritis (AG) is a common disease in humans worldwide. Case definition varies between studies and countries but mostly includes signs and symptoms of diarrhoea, vomiting, nausea, abdominal cramps or pain, fever, and blood or mucus in the stool [ 1 – 5 ]. AG can be caused by a wide variety of pathogens ranging from viruses and bacteria to protozoa and other parasites [ 5 ]. A study in Austria identified norovirus, Clostridium difficile and rotavirus as the most frequent aetiological agents in patients consulting general practitioners (GPs) due to AG [ 4 ]. Norovirus, rotavirus, sapovirus and Campylobacter spp. were the most common organisms among cases of infectious intestinal disease (IID) presenting to the GP in the UK [ 6 ].
Bacterial pathogens causing AG which have to be reported to the National Notification System for Infectious Diseases (NNSID) include positive laboratory tests for Campylobacter spp., Salmonella spp., and Shigella spp. as well as clinical and laboratory reports of positively tested patients with Listeria monocytogenes and enterohaemorrhagic Escherichia coli (EHEC). None of the above-mentioned viral causes of AG are notifiable in Switzerland [ 7 ]. As a result, the NNSID underestimates the true burden of AG because of non-notifiable pathogens causing AG. Additionally, not every patient suffering from AG presents to a physician (under-ascertainment) and, the physician does not always initiate stool diagnosis to investigate the aetiology of the illness (under-reporting) [ 8 , 9 ]. Hence, what is seen in the Swiss mandatory notification system represents only an incomplete picture of the burden of disease due to AG. The determinants of under-ascertainment or under-reporting have been described for several countries but not for Switzerland: In the UK, it is estimated that every case of IID reported to national surveillance represents 9.5 cases presenting to a GP or 147 cases in the community [ 6 ]. In the Netherlands, 8% of patients with an IID visited a physician [ 10 ]. Van Cauteren et al. [ 11 ] estimated that of 115 community cases of campylobacteriosis and 20 community cases of salmonellosis one case is reported to the surveillance system in France. However, it has to be noted that the French surveillance systems are voluntary for these two pathogens.
Swiss routine surveillance data suggest an increasing frequency of campylobacteriosis and a decreasing frequency of salmonellosis [ 12 ]. More than half of campylobacteriosis patients in a case–control study approached a physician within 3 days after onset of symptoms and 14.5% were hospitalised [ 13 ]. A subsequent qualitative survey among primary care physicians described case management approaches including treatment strategies and stool diagnostic testing behaviours from the physicians’ perspective for patients with AG [ 8 ]. Four main approaches were identified of which only two—the “test & wait” and the “test & treat” approaches—include stool specimen testing and, hence, would result in case registration in the mandatory disease surveillance system in case of a positive test outcome. Healthcare costs for AG in Switzerland were estimated at €29–45 million annually [ 14 ].
In Switzerland, we lack data on under-ascertainment and under-reporting. Under-ascertainment refers to people not seeking healthcare and, hence, not being captured by the surveillance system as defined by Gibbons et al. [ 9 ]. Under-reporting is defined as people seeking healthcare but not being reported because of under-diagnosis—not diagnosing or misdiagnosing the infection or pathogen—or under-notification—failure to report positive diagnoses [ 9 ].
This study within the Swiss Sentinel Surveillance Network, Sentinella, aimed at understanding the lower levels of the burden of illness pyramid and addressing the incidence of AG in a broader context. Specifically, the study aimed at understanding determinants of under-diagnosis by (1) estimating the incidence and burden of AG seen at the primary care level, (2) describing the physicians’ case management (diagnostics, treatment) of AG patients and (3) estimating the work loss due to AG of cases presenting to a physician.
A 1-year, longitudinal study in Sentinella, during the year 2014, was conducted asking physicians to report cases of AG on a weekly basis (later referred to as data from the “weekly questionnaire”). A questionnaire about disease characteristics, stool testing, and treating strategies was completed for a subset of cases (later referred to as “supplementary questionnaire”).
Study setting
Sentinella is a voluntary surveillance system and research network of primary care physicians existing since 1986 which is operated and funded by the Federal Office of Public Health (FOPH). Physicians are organised in six geographical regions, each having its representative within the Sentinella steering committee. The steering committee, consisting of physicians and researchers of academic primary care institutes, meets regularly to set the research priorities and to decide on submitted projects. Our study was accepted to run in 2014.
During the Sentinella-year 2014, 172 physicians (47% general practitioners, 37% internists and 16% paediatricians; thereafter referred to as “Sentinella-physicians”) covering entire Switzerland were active in the network. In Switzerland, 6930 physicians were practicing in the ambulatory sector with the main specialty “general internal medicine” (summarising general practitioners and internists) or “paediatrics” in 2014 according to the Swiss medical association FMH [ 15 ]. Among these, 86% were practicing in general internal medicine and 14% in paediatrics.
Case definition
A case of AG was defined as (a) a patient consulting a Sentinella-physician for the first time during the illness episode and suffering from diarrhoea (at least 3 watery or pasty stools daily; for at least 24 h but 14 days the longest) likely due to an infectious cause or (b) a patient consulting a Sentinella-physician for the first time during the illness episode with vomiting and abdominal cramps without significant diarrhoea, likely due to an infectious cause. Patients were excluded if diarrhoea was due to a known gastrointestinal disease (e.g. Crohn’s disease, ulcerative colitis, coeliac disease), medication intake (e.g. antibiotics) or food intolerance. Also patients with persistent diarrhoea (>14 days), or if vomiting was due to pregnancy, were excluded.
Data collection
Sentinella-physicians reported basic data on patients suffering from AG on a weekly questionnaire, and more detailed data for a subsample of patients through a supplementary questionnaire which were available in German and French. German versions of the weekly (part on AG only) and supplementary questionnaires are available online (see Electronic Supplementary Material 1). The questionnaires were piloted with 10 general practitioners.
The weekly questionnaire included information on sex, date of birth, stool testing and hospitalisation of all AG patients (see case definition) seen in the corresponding week. The supplementary questionnaire contained additional questions on employment status, dates of symptom onset and consultation(s), signs and symptoms until first consultation, general condition, antibiotic and symptomatic treatment, stool testing, sick leave, hospitalisation, sequelae, and selected risk exposures in the 7 days preceding symptom onset.
Weekly questionnaires were available on paper and electronically according to the Sentinella standard procedure (method chosen by physician). More than half of the Sentinella-physicians reported electronically, all others reported on paper. Supplementary questionnaires were available on paper only. While weekly paper questionnaires were sent to the FOPH once a week by postal mail according to routine procedures, Sentinella-physicians were asked to send the supplementary questionnaire as soon as they considered the corresponding case as “completed”. Weekly electronic questionnaires were entered directly into the Sentinella-database by the Sentinella-physician.
Information available on Sentinella-physicians included the physicians’ specialty and location of practice. Sentinella-physicians additionally reported the total number of daily physician–patient contacts (PPCs) on the weekly questionnaire. A PPC is defined as each consultation independent of place (in practice or as domiciliary visit) and time (during or off consultation-hour or on emergency service) and serves as denominator for calculating disease incidence rates.
Subsample for supplementary questionnaire
We expected that each Sentinella-physician would report around two AG cases per week based on the pilot testing and discussions with physicians. Assuming that 150 physicians report during 48 weeks, 14,400 cases were expected during the 1-year-study period. To reduce the anticipated work load for Sentinella-physicians but still reaching an appropriate sample size allowing for estimates with acceptable precision, we decided to apply the supplementary questionnaire to a subsample of cases. The targeted subsample size was set at 4800 cases (one-third of all cases). A sampling scheme was defined whereby every Sentinella-physician had to complete supplementary questionnaires during four consecutive weeks four times a year (=16 weeks per physician per year). We randomly assigned each Sentinella-physician a sampling pattern with sampling periods distributed equally over the year, hence not allowing for two consecutive sampling periods.
Case numbers in the first half of the study period were lower than expected necessitating the sampling scheme to change to full sampling. Starting from week 25 (starting on 14.06.2014), supplementary questionnaires had to be completed for every AG patient until the end of the study.
Data entry and analysis
Weekly questionnaires on paper forms and all supplementary questionnaires were entered into the electronic Sentinella database at the FOPH. Ten percent of supplementary questionnaires was randomly selected for double entry to assess data quality. Double entries of questionnaires were compared and discrepancies were eliminated by re-checking against the original paper forms.
Cases of Sentinella-physicians who reported PPC for less than 75% of the weeks during the study period, i.e. <39 of 52 weeks were excluded from data analysis. This rule and cutoff value for regularly reporting physicians are standard for analyses of Sentinella data. Additionally, cases not fulfilling the case definition or cases where the Sentinella-physician spontaneously indicated a final diagnosis not in agreement with infectious AG were excluded from the analysis of supplementary questionnaire data.
Data of weekly questionnaires were analysed descriptively. We calculated the average number of cases per Sentinella-physician and week and the number of initial consultations due to AG per 1000 PPCs per week. Additionally, we estimated the incidence and total number of first consultations due to AG at the primary care level for 2014 in Switzerland by the standard extrapolation of the Sentinella system which is described elsewhere [ 16 ].
Due to the mid-study change in the sampling scheme of supplementary questionnaires, analyses of the supplementary questionnaire data were weighted according to the sampling probability: information from the supplementary questionnaire of cases reported during the first half of the study period was analysed using a sampling weight of 3.25 (as each physician was required to submit a supplementary questionnaire for each case seen during 16 of 52 weeks; 1/(16/52) = 3.25) while information reported during the second half had a sampling weight of 1 (supplementary questionnaire required for every case). Point-estimates including 95% confidence intervals (CI) and interquartile ranges (IQR) for medians are reported for weighted analyses. Data from supplementary questionnaires were analysed descriptively and differences were assessed for significance by weighted, univariable logistic regression. For all analyses involving employment status, only patients aged 15–64 years were considered. Data were analysed and represented graphically using Stata 13.1 (StataCorp.). Maps were created using ArcGIS 10.2.1 for desktop (Environmental Systems Research Institute, Inc., Esri).
Physician and patient characteristics
In total, 3867 cases of AG were reported on weekly questionnaires by 172 participating Sentinella-physicians. After exclusion of cases reported by not regularly reporting Sentinella-physicians (130 cases) and for other reasons (3 cases), 3734 cases were used for analyses of weekly questionnaires. 2200 cases were retained for the analyses of supplementary questionnaires. The detailed inclusion process is described in Fig. 1 .
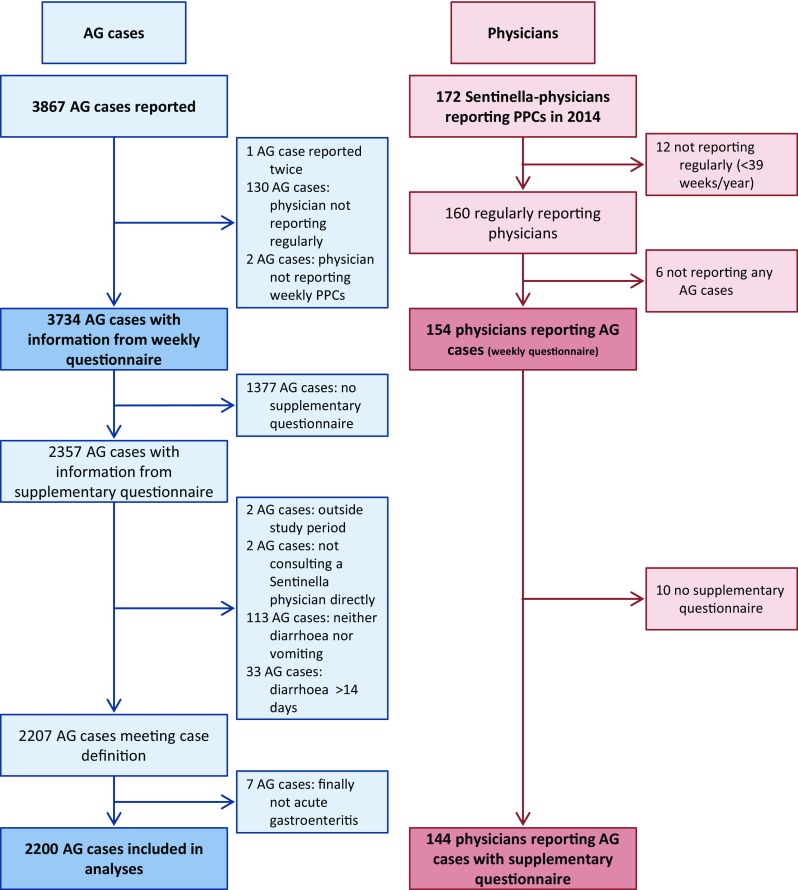
Study profile of notified cases and reporting physicians. Acute gastroenteritis study, Swiss Sentinel Surveillance Network, 2014. AG acute gastroenteritis, PPC physician–patient contact
Out of 172 physicians registered in the Sentinella system in 2014, 154 of the regularly reporting physicians reported at least one case of AG on the weekly questionnaire. Over the whole study period, individual physicians reported up to 400 cases (median 17, IQR 7–29). A total of 144 physicians submitted at least one supplementary questionnaire of a case fulfilling the case definition (Fig. 1 ). The subsample of cases with supplementary questionnaires was comparable to cases reported on weekly forms in terms of basic patient characteristics (Table 1 ).
Basic characteristics of acute gastroenteritis cases reported on the weekly and supplementary questionnaires by physicians from the Swiss Sentinel Surveillance Network in 2014
Median age of AG cases was 21 years (IQR 5–41 years). Children, adolescents and young adults (age groups <1, 1–4, 5–9, 10–14, 15–19 and 20–29 years) were overrepresented among AG cases consulting a physician compared to the frequency of those age groups in the general Swiss population for both genders (Fig. 2 ). In the age group of 10–14 year olds, males were more frequent than females. In adults, female cases aged 20–29 years were most frequently reported while in males the 30–44 year age group predominated.
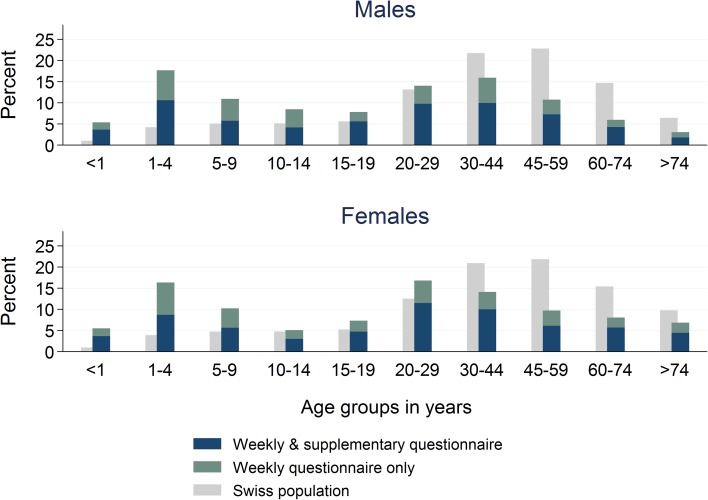
Age distribution by sex among acute gastroenteritis cases reported by Sentinella-physicians on weekly and/or supplementary questionnaires. Swiss Sentinel Surveillance Network, 2014; age distribution of Swiss population (official numbers [ 17 ]) added for comparison
Burden of AG at primary care level
Each week, 15–139 cases (median 69, IQR 54–80) were reported (Fig. 3 ). Case numbers were highest during the first weeks of the year (maximum in week 4) and decreased thereafter. A median rate of 5.4 first consultations due to AG per 1000 PPCs per week (IQR 4.6–6.7) was observed. The notifications correspond to 2146 first consultations due to AG at primary care level per 100,000 inhabitants or 174,610 first consultations due to AG in Switzerland in 2014 using the standard extrapolation method of the FOPH for Sentinella data. Incidence (of first consultations) by Sentinella-region is displayed in Fig. 4 .
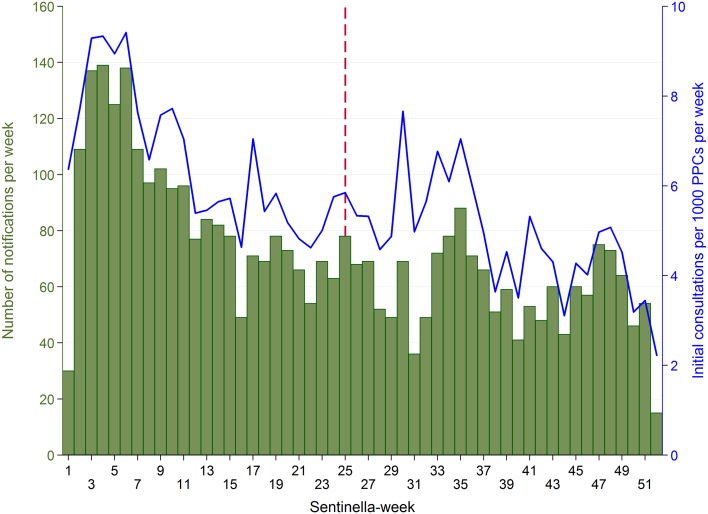
Acute gastroenteritis cases reported by physicians from the Swiss Sentinel Surveillance Network in 2014 (28.12.2013–26.12.2014): weekly case numbers ( bars ) and number of initial AG consultations per 1000 physician–patient contacts (PPCs, “consultations”) per week ( line ). Vertical , dashed line date of change of sampling scheme (from subsample of cases with supplementary questionnaires to supplementary questionnaire for every reported case)
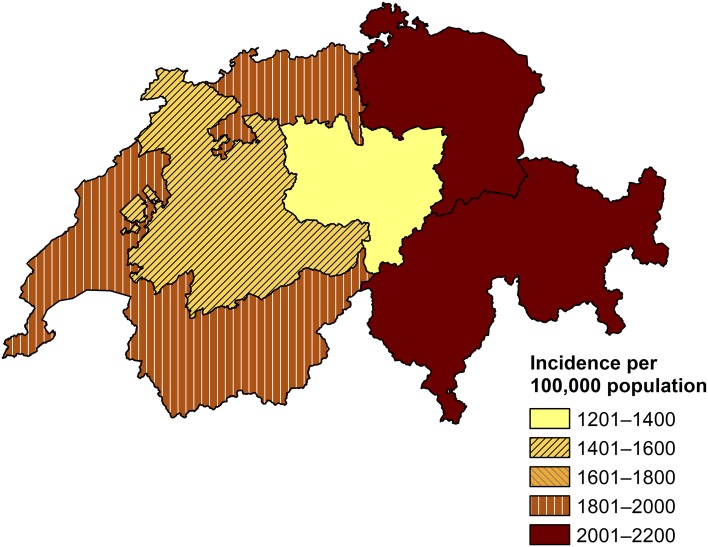
Calculated incidence of first consultations due to acute gastroenteritis at primary care level in Switzerland by Sentinella-region, based on standard extrapolation. Swiss Sentinel Surveillance Network, 2014. Note: an outlier (one physician reporting 400 cases) was excluded from this extrapolation by region. Source of map shapefile: Swiss Federal Office of Topography
Health care seeking and clinical presentation
The median time from symptom onset to first consultation was 2 days (95% CI 2.0–2.0, IQR 1.0 [95% CI 1.0–1.0]–3.0 [95% CI 2.4–3.6]). The majority of patients (87.9% [95% CI 85.6–89.9]) suffered from diarrhoea (Table 2 ). Loss of appetite was reported for 63.5% (95% CI 58.4–68.4), abdominal pain or cramps for 61.1% (95% CI 57.0–65.1), nausea for 60.4% (95% CI 56.6–64.1) and vomiting for 57.5% (95% CI 54.3–60.7) of patients. Less frequently reported signs and symptoms included flatulence, fever, dehydration and headache.
Characteristics of cases with acute gastroenteritis at first consultation and number of consultations as reported by primary care physicians from the Swiss Sentinel Surveillance Network, 2014
a Multiple answers possible
The majority of patients consulted the Sentinella-physician only once (79.6%, 95% CI 76.5–82.4) (Table 2 ). The median general condition of cases as reported by Sentinella-physicians at the time of first consultation was 7 (95% CI 6.5–7.5, IQR 5.0 [95% CI 4.5–5.5]–9.0 [95% CI 8.5–9.5]) on a rating scale from 1 (poor) to 10 (good). Overall, 86.3% (95% CI 83.1–89.0) of employed patients were unable to work. The odds for a good general condition (7 or above) was lower for employed patients compared to unemployed patients although not significantly (odds ratio [OR] 0.76, 95% CI 0.52–1.11, p = 0.159). The median duration of sick leave was 4 days (95% CI 3.8–4.2, IQR 3.0 [95% CI 3.0–3.0]–5.0 [95% CI 4.5–5.5]). For all except seven cases, the duration of sick leave was below 15 days.
The hospitalisation rate was 2.7% (95% CI 1.9–3.7). The highest hospitalisation rate was observed for the >74 year age group (11.5%, 95% CI 6.4–19.9) whereas for the remaining age groups the rates were below 4%. For 2.0% (95% CI 1.4–2.9) of patients, Sentinella-physicians reported sequelae, like dehydration, diverticulitis, or colitis. No deaths due to AG were reported.
Stool diagnostics and results
Sentinella-physicians reported the initiation of stool specimen testing in 12.3% (95% CI 10.1–14.8); in 11.6% (95% CI 9.5–14.1) of cases they indicated that the sample was actually sent off (Table 3 ). The odds for stool testing did not differ between sexes ([female vs. male]: OR 1.13, 95% CI 0.84–1.50, p = 0.423) but differed by age group ( p < 0.001): The proportion of stool testing was generally higher among older age groups. Paediatricians initiated stool testing less frequently (OR 0.32, 95% CI 0.18–0.55, p < 0.001) than general practitioners. The odds of initiating stool testing did not differ significantly for internists compared to general practitioners (OR 1.13, 95% CI 0.71–1.78, p = 0.610).
Frequency of and reasons for prescription of stool diagnostics among acute gastroenteritis patients consulting primary care physicians from the Swiss Sentinel Surveillance Network, 2014
a Two pathogens were identified in 11.5% (95% CI 5.4–22.9) of the 98 cases with a positive stool test result
Even though the questionnaire explicitly asked for the main reason for initiating stool testing, multiple answers were given for 31.0% (95% CI 24.9–37.8) of cases. The three most frequent reasons mentioned were protracted course of disease (29.4%, 95% CI 21.9–38.2), poor general condition (11.5%, 95% CI 6.9–18.4) and due to a specific symptom (9.5%, 95% CI 4.6–18.6) when excluding those with multiple answers. When considering also multiple answers, staying abroad before symptom onset was the third most frequent reason (data not shown).
Travelling within the 7 days preceding symptom onset was reported for 9.0% (95% CI 7.4–10.8) of cases. Patients with recent travel history were significantly more likely to undergo stool testing than patients not reporting any recent travels (OR 3.60, 95% CI 2.47–5.33, p < 0.001). Among patients with recent travel history, 30.0% (95% CI 22.7–38.6) were tested while for patients without travel to a foreign country in the 7 days preceding the symptom onset this proportion was 10.6% (95% CI 8.6–13.0). “Staying abroad” was indicated as the main reason for testing for 40.8% (95% CI 24.4–59.6) of patients with a travel history. Protracted course of disease was the second most often mentioned reason for stool testing among patients with travel history abroad (17.4%, 95% CI 7.2–36.2).
A positive test result was reported for more than one-third (35.9%, 95% CI 29.2–43.2) of tested patients while for the remaining 64.1% (95% CI 56.8–70.8) of patients test results were negative or not specified. The most frequently identified pathogen was Campylobacter spp. (50.8%, 95% CI 39.2–62.3) followed by norovirus (10.9%, 95% CI 5.0–21.9), and Blastocystis spp. (9.6%, 95% CI 4.0–21.1) (Table 3 ). Other pathogens identified included rotavirus, Clostridium spp., Entamoeba spp., pathogenic E. coli , Candida spp., Salmonella spp., Giardia spp., microsporidia, adenovirus, Aeromonas spp. and hepatitis E virus. Two pathogens were identified in 11.5% (95% CI 5.4–22.9) of the 98 cases with a positive stool test result.
Approaches for symptomatic and antibiotic therapy
In 92.0% (95% CI 89.8–93.8) of cases, Sentinella-physicians gave dietary recommendations, or prescribed symptomatic and/or antibiotic treatment. Most commonly, patients were advised to care for fluid replacement by the intake of sufficient tea, broth etc. (58.3%, 95% CI 53.0–63.3) (Table 4 ). Distinct rehydration therapies such as electrolyte solution (11.4%, 95% CI 7.8–16.4) and infusion therapies (1.7%, 95% CI 1.1–2.6) were less frequently prescribed. Symptomatic treatment included probiotics (45.9%, 95% CI 39.1–52.8), antiemetics (45.4%, 95% CI 40.5–50.4), antidiarrhoeals (28.8%, 95% CI 23.6–34.6), analgesics (16.3%, 95% CI 12.8–20.5), and spasmolytics (15.0%, 95% CI 11.5–19.2). Antibiotics were prescribed in 8.5% (95% CI 6.5–11.0) of cases (Table 4 ).
Frequency of prescription of antibiotic and symptomatic treatment, and reasons for prescription of antibiotic therapy among acute gastroenteritis patients consulting primary care physicians from the Swiss Sentinel Surveillance Network, 2014
The Sentinella-physicians initiated stool testing and prescribed antibiotics at the first consultation in 33 cases (unweighted results, Table 5 ). Stool diagnostics revealed the presence of a pathogen susceptible to antibiotics in 20 of these cases. No antibiotics were prescribed in 22 cases even though a pathogen which is theoretically susceptible to antibiotics was identified.
Time point of prescription of stool testing and antibiotic treatment among acute gastroenteritis patients consulting primary care physicians, Swiss Sentinel Surveillance Network, 2014
Unweighted results. Cases with missing information on (date of) antibiotic prescription and/or (date of) stool test were excluded
a Not considering possible antibiotic resistances and treatment recommendations
The majority of patients receiving antibiotics was treated with quinolones (60.2%, 95% CI 48.5–70.9), followed by macrolides, metronidazole, aminopenicillin, trimethoprim/sulfamethoxazole, cephalosporin and tetracycline (Table 4 ). Two or more antibiotic classes were reported to be used for 8.5% (95% CI 4.6–15.2) of cases. No antibiotic class was reported for 1.6% (95% CI 0.6–4.4) of cases treated with antibiotics.
Main reasons for the prescription of antibiotic therapy included (suspicion of) bacterial gastroenteritis (41.1%, 95% CI 25.0–59.5), duration of illness (9.0%, 95% CI 3.4–19.6), a specific symptom (7.2%, 95% CI 3.4–14.8) and others (Table 4 ). Sentinella-physicians mentioned several reasons for 23.9% (95% CI 16.6–32.2) of the patients despite being asked to indicate only the main reason. When considering also multiple answers, “poor general condition” was the third most frequently mentioned reason for antibiotic therapy (data not shown).
Similar to stool testing, antibiotic prescription was associated with age ( p < 0.001) and with the physicians’ specialty ( p < 0.001) but not with sex ( p = 0.511) (data not shown). Again, children and adolescents were less frequently treated with antibiotics compared to adults. Among the >74-year-old age group, one-fifth of cases received antibiotics (20.0%, 95% CI 12.8–29.7). Nearly three-quarter of the antibiotic therapies were prescribed at the first consultation (71.3%, 95% CI 60.5–80.1). These patients had a lower general condition according to physicians’ impression (median 5.0, 95% CI 4.0–6.0, IQR 4.0 [95% CI 3.0–5.0]–7.0 [95% CI 6.0–8.0]) than patients receiving antibiotics later on (median 7.0, 95% CI 6.0–8.0, IQR 5.0 [95% CI 4.0–6.0]–8.0 [95% CI 7.0–9.0]) and also suffered slightly more frequently from fever (44.7%, 95% CI 34.5–55.4 vs. 38.9%, 95% CI 24.0–56.2). However, both differences were not statistically significant. Patients with a recent history of travel had significant higher odds to undergo antibiotic therapy (OR 1.75, 95% CI 1.06–2.88, p = 0.029).
This study underscored that acute gastroenteritis is common in Swiss primary care: extrapolated annual consultation numbers (175,000 first consultations) are comparable to those of influenza-like illness (ILI) during an influenza season (varying between 107,000 and 276,000 ILI cases in the last three seasons [ 18 – 20 ]). The majority of patients is symptomatically treated and does not require multiple consultations. However, most episodes of AG lead to a sick leave of several days, though the physician-assessed general state of the patients is considered as “fairly good”. Stool specimen testing is not systematically conducted and antibiotic therapy is applied to less than 10% of patients.
Multiple factors influence physicians’ decision making
Sentinella-physicians reported more than one reason for stool testing in a third of cases despite being explicitly asked for the main reason in the questionnaire. This suggests that a combination of factors plays a role in decision making. The same holds true for the prescription of antibiotic treatment where in around a quarter of cases several reasons were mentioned albeit physicians were asked to indicate the main reason. The reasons mentioned most frequently for stool testing—namely protracted course of disease, poor general condition, due to a specific symptom and a history of recent travel—are in line with findings from other studies: three of the aforementioned four factors (all except “specific symptom”) were also mentioned by GPs participating in a qualitative study in Switzerland [ 8 ] and in a study from Northern Ireland and the Republic of Ireland [ 21 ]. The Irish study further reported that stool testing is frequently prescribed if the illness is associated with an outbreak or if the physicians suspect a link with a particular consumed food item or food premises (pub, restaurant, take away). Similarly, a qualitative study among GPs in the UK found that long duration of illness, recent travel, blood in the stool, patient being unwell and exclusion of an infectious cause were the reasons mentioned most frequently for stool testing [ 22 ]. Factors most strongly associated with requesting a stool culture were bloody diarrhoea, diarrhoea lasting more than 3 days, and a diagnosis of AIDS in a postal survey among physicians in the US [ 23 ].
Considering that protracted course of disease and poor general condition were mentioned most frequently as main reasons for stool testing in our study, the difference in reported general condition at the time of first consultation among tested and untested patients seems rather small (median 7.0, 95% CI 6.5–7.5, IQR 5.0 [95% CI 4.5–5.5]–8.0 [95% CI 7.5–8.5] vs. median 8.0, 95% CI 7.5–8.5, IQR 6.0 [95% CI 5.5–6.5]–9.0 [95% CI 8.5–9.5]). One explanation for this is that a “protracted course of disease” does not necessarily equate with a poor general condition but simply reflects the lack of improvement of symptoms with an average or fairly good general condition. Most of the aforementioned studies [ 8 , 21 , 22 ] acknowledge that decisions for testing are subjective and depend on the physicians’ experiences and attitudes.
AG, whether of viral or bacterial origin, is usually self-limiting [ 5 ]. Antibiotics are mainly recommended for severely affected patients and are most effective if given early [ 5 , 24 , 25 ]. “Bacterial gastroenteritis” was most frequently mentioned as main reason for antibiotic therapy in our study. We cannot judge whether this reasoning was based on laboratory results or on physicians’ experience. However, only two cases with positive stool test results for pathogens not susceptible to antibiotics were prescribed antibiotics in our study. The second most common reasoning for antibiotic treatment, namely duration of illness, was also reported by Swiss GPs in an extensive qualitative assessment [ 8 ]. A study from Poland concluded that factors associated with antibacterial drug administration included the work environment of the physician (working in large practices and hospital wards favoured antibiotic prescription compared to small practices), presence of fever, or mucus or blood in stool, age of the patient and (rural/urban) residence [ 26 ]. The presence of fever, or mucus or blood in stool could also be a factor leading to antibiotic therapy in our study as the third most frequent mentioned main reason for antibiotic prescription was suffering from a specific symptom.
Some 62% of all cases with a laboratory-confirmed Campylobacter infection received antibiotic treatment in our study. This finding is important in the context of antibiotic resistance development. More than half of those patients received quinolones and one-third was treated with macrolides—a finding confirming results from an earlier qualitative study among Swiss GPs [ 8 ]. Given antibiotic resistance levels for fluoroquinolones as high as 55.3% for human Campylobacter isolates in Switzerland in 2014 [ 27 ], these studies’ findings underscore the need for changes in prescription practise in Switzerland. A similar level of resistance (60.2%) was observed in Europe in 2014 [ 28 ]. Consequently, the European Food Safety Authority and the European Centre for Disease Prevention and Control do no longer recommend fluoroquinolones for the empirical treatment of human campylobacteriosis.
Physicians’ case management impacts on the mandatory surveillance system
A stool test was performed only for 11.6% of patients consulting a Sentinella-physician due to AG. Of these, 19.8% (95% CI 15.1–25.6) had a positive result for a notifiable pathogen. Hence, a very small proportion of 2.3% (=11.6 × 19.8%) of AG patients consulting a Sentinella-physician were actually reportable to the mandatory reporting system. This is in line with Swiss physicians’ typical treatment pattern for AG of “wait & see”, which can be followed by a “treat & see” approach or a desirable (from the perspective of the NNSID) “test & see” or “test & treat” approach based on illness progression [ 8 ]. Considering the (main) reasons mentioned for stool testing, patients with a prolonged duration of illness and patients reporting recent travel abroad are likely overrepresented among notified cases. The proportion of patients with stool testing varies substantially between countries: it was found to be 4.3 or 9.1% in France [ 29 ], 6% in Italy [ 30 ], 7% in Ireland [ 31 ], 12% in the Netherlands [ 32 ], 19% in the US [ 33 ] and 25% in Denmark [ 34 ].
The pathogen most often identified through stool testing in this study ( Campylobacter spp.) is also the pathogen most frequently reported to Swiss national surveillance. Norovirus, which is not notifiable in Switzerland but in several countries of the European Union, was the second most common identified pathogen.
Mild disease with high socio-economic burden
Physicians rated the general condition of AG patients as relatively good. Nevertheless, a high proportion of 86.3% of employed patients was not able to work due to the illness. Sick leave is considerable with a median of 4 days. The risk of transmission seems to play a subordinate role as a reason for inability to work. Similar findings were reported in a French study where 79% of working patients were on sick leave for a median duration of 3 days [ 35 ]. In a Danish study, only 35% of patients with AG reported having missed work or school as a result of illness [ 34 ]. However, this Danish study was a population-based study in which only 13% of patients were seen by a physician and/or hospitalised. In our study, we did not observe a difference in time from symptom onset to consultation between employed and unemployed patients (data not shown). This indicates that the need of a medical certificate is unlikely to be a main reason for consultation.
It is well known that some pathogens causing AG are easily transmitted from human-to-human, especially viruses, and contact with diarrhoea patients has been described as a risk factor for AG previously [ 35 , 36 ]. In our study, 28.6% (95% CI 24.9–32.6) of the patients had contact to other people suffering from similar signs and symptoms in the 7 days preceding symptom onset. Thus, it is possible that these patients had a common source of infection or transmitted the disease among each other.
In summary, our findings suggest that AG is a common, but generally mild disease which results, however, in a high social and economic burden. The overall financial burden due to AG (including losses in productivity) is likely a multiple of the healthcare costs estimated for Switzerland in the range of €29–45 million annually [ 14 ].
Sentinella is invaluable to investigate current public health issues
All information for this study was derived from physicians in the Swiss Sentinel Surveillance Network. This study was specifically set up by the FOPH to clarify current epidemiological questions about gastroenteritis in Switzerland, using a national primary care sentinel surveillance platform.
We consider it a strength of the study to have obtained information on diagnosis and treatment directly from treating primary care physicians. However, the actual duration of sick leave might have been longer or shorter than reported or certified by the physician. Similarly, we could not record the overall duration of the illness as in this study we could not send out follow-up questionnaires at the end of an AG episode.
A limitation of our study is the change in sampling scheme for supplementary questionnaires for the second half of the study period, especially considering that AG is subject to seasonal variation. However, we believe that changing to full sampling and using weighted analyses to adjust for the change in sampling scheme resulted in more reliable data than continuing without changing the sampling scheme and obtaining far less supplementary questionnaires.
We expected to observe a seasonality of case reports considering the literature [ 4 , 36 ], results of a previous study [ 8 ] and surveillance data [ 12 ], with a peak of AG in winter (December–March) and during summer (June–September). Instead we found a decreasing number of initial consultations per 1000 PPCs over the year which we assume is partially due to reporting fatigue of the Sentinella-physicians partaking in the study. This is supported by a survey conducted among Sentinella-physicians in which they were asked about the time required for participating in the sentinel network—in total and for the different research topics. Physicians indicated that the study on AG was comparatively time-consuming although the majority indicated that the total amount of time required for notifying was acceptable [ 37 ].
Not to our complete surprise, this study has shown that acute gastroenteritis is a common disease in Switzerland with consultation frequencies comparable to influenza-like illnesses. AG presented to physicians lead to substantial sick leave in the employed, resulting in considerable socio-economic costs due to productivity loss.
Furthermore, as suspected, the study confirms that the National Notification System for Infectious Diseases captures—if at all—only a fraction of the scope of the problem (see introduction for currently notifiable diarrhoea-causing pathogens). Hence, the Swiss Sentinel Surveillance Network, Sentinella, represents a very important complementary surveillance instrument to grasp principal dynamics of infectious disease epidemiology at the primary care level.
The FOPH and the Federal Food Safety and Veterinary Office, being responsible to maintain population health and food safety in Switzerland, are currently lacking effective tools for pinpointing and a comprehensive national programme addressing the control of foodborne diseases and AG. While there are efforts to increase food safety and consumer hygiene including campaigns to increase awareness for food and kitchen hygiene among consumers in Switzerland, prevention measures to reduce contamination at food production or retail level are incomplete. Overall, there is an imbalance in national disease prevention and control efforts for AG considering that national strategies to reduce the burden of seasonal influenza—an infection with a disease burden comparable to AG—exist since many years.
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all physicians who contributed and provided feedback to pre-testing the questionnaire, especially Professor Andreas Zeller and Dr. Christoph Merlo. Albeit being formally listed in globo as co-authors, all the physicians constituting the Swiss Sentinel Surveillance Network deserve a special word of thanks for relentlessly contributing to the network and collaborating with us weekly in this 1-year study. The authors gratefully acknowledge the help of the Notification Systems Unit of the Federal Office of Public Health, especially Ms. Diana Guido and Dr. Raphael Rytz, for their help in preparing the study and during data collection. The statistical support of Dr. Jan Hattendorf (Swiss Tropical and Public Health Institute) is gratefully acknowledged. We thank the Federal Office of Public Health, Bern, Switzerland for funding this study.
Compliance with ethical standards
Ethical statement.
This study was conducted under the Swiss Epidemics Act (SR 818.101) and the ordinance on disease notification of humans (SR 818.141.1).
Conflict of interest
This study was funded by the Federal Office of Public Health, Bern, Switzerland (Grant numbers 13.004570, 14.000710 and 15.007090). MJ and MM are on the staff of the Federal Office of Public Health and participated in their capacities as public health specialists and their function as scientific collaborators within the organisation.
Claudia Schmutz and Philipp Justus Bless contributed equally to this paper.
- 1. Majowicz SE, Hall G, Scallan E, Adak GK, Gauci C, Jones TF, et al. A common, symptom-based case definition for gastroenteritis. Epidemiol Infect. 2008;136:886–894. doi: 10.1017/S0950268807009375. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. de Wit MA, Hoogenboom-Verdegaal AM, Goosen ES, Sprenger MJ, Borgdorff MW. A population-based longitudinal study on the incidence and disease burden of gastroenteritis and Campylobacter and Salmonella infection in four regions of the Netherlands. Eur J Epidemiol. 2000;16:713–718. doi: 10.1023/A:1026754218713. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Kuusi M, Aavitsland P, Gondrosen B, Kapperud G. Incidence of gastroenteritis in Norway—a population-based survey. Epidemiol Infect. 2003;131:591–597. doi: 10.1017/S0950268803008744. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Huhulescu S, Kiss R, Brettlecker M, Cerny RJ, Hess C, Wewalka G, et al. Etiology of acute gastroenteritis in three sentinel general practices, Austria 2007. Infection. 2009;37:103–108. doi: 10.1007/s15010-008-8106-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Morgan DR, Chidi V, Owen RL. Gastroenteritis. In: Schlossberg D, editor. Clinical infectious disease. 2. Cambridge: Cambridge University Press; 2015. pp. 334–341. [ Google Scholar ]
- 6. Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Das Eidgenössische Departement des Innern. Verordnung des EDI über die Meldung von Beobachtungen übertragbarer Krankheiten des Menschen vom 01. Dezember 2015. Stand am 5. März 2016 (SR 818.101.126). [Ordinance of the FDHA on notification of observations on communicable diseases of human beings of 01 December 2015. Status as of 5 March 2016; in German, French and Italian]. https://www.admin.ch/opc/de/classified-compilation/20151622/index.html . Accessed 24 Aug 2016.
- 8. Bless PJ, Muela Ribera J, Schmutz C, Zeller A, Mäusezahl D. Acute gastroenteritis and campylobacteriosis in Swiss primary care: the viewpoint of general practitioners. PLoS One. 2016;11:e0161650. doi: 10.1371/journal.pone.0161650. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Gibbons CL, Mangen MJJ, Plass D, Havelaar AH, Brooke RJ, Kramarz P, et al. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. 2014;14:147. doi: 10.1186/1471-2458-14-147. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Doorduyn Y, Van Pelt W, Havelaar AH. The burden of infectious intestinal disease (IID) in the community: a survey of self-reported IID in The Netherlands. Epidemiol Infect. 2012;140:1185–1192. doi: 10.1017/S0950268811001099. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Van Cauteren D, De Valk H, Sommen C, King LA, Jourdan-Da Silva N, Weill FX, et al. Community incidence of campylobacteriosis and nontyphoidal salmonellosis, France, 2008–2013. Foodborne Pathog Dis. 2015;12:664–669. doi: 10.1089/fpd.2015.1964. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Schmutz C, Mäusezahl D, Jost M, Baumgartner A, Mäusezahl-Feuz M. Inverse trends of Campylobacter and Salmonella in Swiss surveillance data, 1988–2013. Eurosurveillance. 2016;21:30130. doi: 10.2807/1560-7917.ES.2016.21.6.30130. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Bless PJ, Schmutz C, Suter K, Jost M, Hattendorf J, Mäusezahl-Feuz M, et al. A tradition and an epidemic: determinants of the campylobacteriosis winter peak in Switzerland. Eur J Epidemiol. 2014;29:527–537. doi: 10.1007/s10654-014-9917-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Schmutz C, Mäusezahl D, Bless PJ, Hatz C, Schwenkglenks M, Urbinello D. Estimating healthcare costs of acute gastroenteritis and human campylobacteriosis in Switzerland. Epidemiol Infect. 2017;145:627–641. doi: 10.1017/S0950268816001618. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Foederatio Medicorum Helveticorum. FMH-Ärztestatistik. Berufstätige Ärzte nach Hauptfachgebiet. FMH-Generalsekretariat. 2014. http://aerztestatistik.myfmh2.fmh.ch/ . Accessed 03 Jan 2017.
- 16. Altpeter E, Zimmermann H, Oberreich J, Péter O, Dvořák C, Swiss Sentinel Surveillance Network Tick related diseases in Switzerland, 2008 to 2011. Swiss Med Wkly. 2013;143:w13725. doi: 10.4414/smw.2013.13725. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Bundesamt für Statistik. STAT-TAB: Die interaktive Statistikdatenbank. Swiss Federal Statistical Office, Neuchâtel. 2016. http://www.pxweb.bfs.admin.ch . Accessed 31 Aug 2016.
- 18. Bundesamt für Gesundheit. Saisonbericht Grippe 2015/16. BAG Bulletin. 2016;37.
- 19. Bundesamt für Gesundheit. Saisonale Grippe 2013/14: Epidemiologie, Virologie, Impfstoffversorgung und -zusammensetzung. BAG Bulletin. 2014;27.
- 20. Bundesamt für Gesundheit. Saisonale Grippe 2014/15: Epidemiologie, Virologie, Impfstoffversorgung und -zusammensetzung. BAG Bulletin. 2015;28.
- 21. Scallan E, Fitzgerald M, Cormican M, Smyth B, Devine M, Daly L, et al. The investigation of acute gastroenteritis in general practice: a survey of general practitioners in Northern Ireland and Republic of Ireland. Eur J Gen Pract. 2005;11:136–138. doi: 10.3109/13814780509178257. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. McNulty CA, Lasseter G, Newby K, Joshi P, Yoxall H, Kumaran K, et al. Stool submission by general practitioners in SW England—when, why and how? A qualitative study. BMC Fam Pract. 2012;13:77. doi: 10.1186/1471-2296-13-77. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Hennessy TW, Marcus R, Deneen V, Reddy S, Vugia D, Townes J, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38:S203–S211. doi: 10.1086/381588. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–351. doi: 10.1086/318514. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370:1532–1540. doi: 10.1056/NEJMra1301069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Stefanoff P, Rogalska J, Czech M, Staszewska E, Rosinska M. Antibacterial prescriptions for acute gastrointestinal infections: uncovering the iceberg. Epidemiol Infect. 2013;141:859–867. doi: 10.1017/S0950268812001173. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Swiss Centre for Antibiotic resistance. Antibiotic resistance data. 2016. http://anresis.ch/index.php/Interactive-database.html . Accessed 16 Nov 2016.
- 28. European Food Safety Authority, European Centre for Disease Prevention and Control The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA J. 2016;14:4380. doi: 10.2903/j.efsa.2018.5182. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Van Cauteren D, Turbelin C, Fonteneau L, Hanslik T, De Valk H, Blanchon T. Physician practices in requesting stool samples for patients with acute gastroenteritis, France, August 2013–July 2014. Epidemiol Infect. 2015;143:2532–2538. doi: 10.1017/S0950268814003884. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Scavia G, Baldinelli F, Busani L, Caprioli A. The burden of self-reported acute gastrointestinal illness in Italy: a retrospective survey, 2008–2009. Epidemiol Infect. 2012;140:1193–1206. doi: 10.1017/S0950268811002020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Scallan E, Fitzgerald M, Collins C, Crowley D, Daly L, Devine M, et al. Acute gastroenteritis in northern Ireland and the Republic of Ireland: a telephone survey. Commun Dis Public Health. 2004;7:61–67. [ PubMed ] [ Google Scholar ]
- 32. van den Brandhof WE, Bartelds AI, Koopmans MP, van Duynhoven YT. General practitioner practices in requesting laboratory tests for patients with gastroenteritis in the Netherlands, 2001–2002. BMC Fam Pract. 2006;7:56. doi: 10.1186/1471-2296-7-56. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Scallan E, Jones TF, Cronquist A, Thomas S, Frenzen P, Hoefer D, et al. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathog Dis. 2006;3:432–438. doi: 10.1089/fpd.2006.3.432. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Müller L, Korsgaard H, Ethelberg S. Burden of acute gastrointestinal illness in Denmark 2009: a population-based telephone survey. Epidemiol Infect. 2012;140:290–298. doi: 10.1017/S0950268811000471. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Arena C, Amoros JP, Vaillant V, Ambert-Balay K, Chikhi-Brachet R, Jourdan-Da Silva N, et al. Acute diarrhea in adults consulting a general practitioner in France during winter: incidence, clinical characteristics, management and risk factors. BMC Infect Dis. 2014;14:574. doi: 10.1186/s12879-014-0574-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Karsten C, Baumgarte S, Friedrich AW, von Eiff C, Becker K, Wosniok W, et al. Incidence and risk factors for community-acquired acute gastroenteritis in north-west Germany in 2004. Eur J Clin Microbiol Infect Dis. 2009;28:935–943. doi: 10.1007/s10096-009-0729-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Auswertung der Umfrage zum Meldeaufwand in Sentinella. Sentinella-News. 2014;3:5–9. http://www.sentinella.ch/de/news/media/0b36a3a5956842e2bb79f2d54d8749aa . Accessed 30 Aug 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (1.9 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Mehrdad Mohammadi
We cordially invite you to join our board..
Your valuable suggestions improve our journal quality. We welcome you to be part of our board members.
The Case Report of HBoV Acute Gastroenteritis in a Two-Month- Old Infant
Author information.
- * Mehrdad Mohammadi: Department of Medical Microbiology and Immunology, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran.
- Niloofar Sabzi: Department of Medical Microbiology and Immunology, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran.
- Nikou Bahrami: Department of Biology, Faculty of Science, Shahid chamran University of Ahvaz, Ahvaz, Iran.
- Jul 25, 2020 |
- Volume: 1 |
- Views: 3827 |
- Downloads: 1471
- Download PDF
Introduction: The Human bocavirus was identified from the nasopharyngeal aspirate specimen in 2005, that it includes four subtypes (HBoV 1–4). The HBoV-1 is a major subtype in children’s acute respiratory infections, and others (HBoV 2–4) were in stool specimens. The pathogenic role of Human bocavirus 2–4 in acute Gastroenteritis has not confirmed yet, and therefore all of the reports were done to confirmation of this issue.
Case presentation: In this report, we have a 2-month-old boy with acute Gastroenteritis admitted to Shahid Beheshti hospital of Kashan, Iran. The stool sample of the patient was tested for Human bocavirus by PCR of the NP-1 gene. The other major gastrointestinal pathogens of Salmonella spp . And Shigella sp p , Giardia lamblia and Entamoeba histolytica confirmed by specialized microbiological procedures and viral pathogen of Rotavirus by ELISA technique. This case confirmed by HBoV-NP-1 positive control of cloned plasmid. All of the clinical manifestations were analyzed by pediatric nursing in the admission period in the hospital.
Conclusions: This case has been positive for Human bocavirus by PCR of the NP-1 gene of 354 bp. The major signs were diarrhea, fever, dehydration, and abdominal pain. This case dismissed the hospital after supportive therapies for dehydration. We show an important confidential chance to Human bocavirus that can be a gastrointestinal pathogen in pediatric patients and make severe diarrhea in young children. This conclusion needs further analysis in widespread studies.
Introduction
The Human Bocavirus (HBoV) was identified from a nasopharyngeal aspirate specimen in Sweden by Allander in 2005, that it includes four subtypes (HBoV 1–4) [1,2] . The HBoV-1 is a major subtype in children’s acute respiratory infections, and others (HBoV 2–4) were in stool specimens [3] . The HBoV is wide-spread infections world-wide with the main prevalence of younger than three years old in children [4] . This virus is supposed to be a respiratory infection pathogen currently, but not a gastrointestinal infection pathogen, not yet [5,6] . Although most reports report this virus in fecal samples in acute diarrhea of children, that is not confirmed as a gastrointestinal pathogen [4,6] . Because of limitations in HBoV culture and animal examinations, forgive a pathogenic role to HBoV need to clinical studies and doing these reports in hospitals [4–6] .
Case Presentation
The present study was performed according to the Helsinki Declaration (Ethical Principles for Medical Research Involving Human Subjects). The research board approved it of the Ethics Committee of the Capital Institute of Pediatrics, Kashan University of Medical Sciences, Iran.
All patient data were anonymously reported. A 2-month-old boy with acute Gastroenteritis admitted to Shahid Beheshti Hospital of Kashan, Iran. There were vomiting, alternating fever, and yellow-watery feces in historical and clinical examinations in 2-days ago. This infant’s parents were reports 10–15 watery stools in the previous 48 hours, and he was quiet and pale seriously. The nursing home was protected from the infant at home every day, and his diet was milk powder only. The clinical examinations have been listed in (Table 1) .
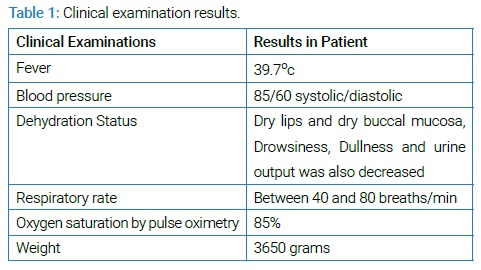
Laboratory tests showed hemoglobin 11.6 g/dl, leukocytosis with 19800/mm 3 WBC (Lymphocyte 83%), Platelets 496000/mm 3 , C-reactive protein was positive, the erythrocyte sedimentation rate was 59, sodium concentration of 136 mEq/L and the fecal culture of Salmonella spp and Shigella spp . were negative. The stool analysis was a Yellow-Watery specimen with many WBC without any RBC and no cysts and trophozoite of Giardia lamblia and Entameoba histolytica and no eggs of worms. The stool specimen was negative for Rotavirus by the ELISA test.
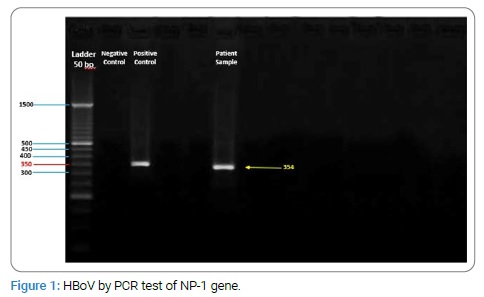
In contrast of the above tests, was positive for HBoV by PCR test of NP-1 gene [7] . The HBoV detection was 354 bp fragment of NP-1 gene and confirmed by positive control of NP-1 Cloned plasmid from Tehran University of Medical Sciences (Figure 1) .
This patient obtained intravenous fluid therapy to understand better the above conditions—a solution containing dextrose 5% plus electrolytes. Digestive losses of water and electrolytes were compensated by Oral Rehydration Solution (ORS). For prevention of adding bacterial infections in hospital antibiotic treatment was done by Ceftriaxone 100 mg/Kg/day to three days. The patient was discharged from the hospital four days later.
Acute gastroenteritis in infants has the most viral and bacterial causal agents like Salmonella , Shigella , Campylobacter , Rotavirus, Norovirus, Giardia , Entamoeba histolytica , et al. [8] . The Human bocavirus represented as an acute respiratory infection agent currently, but that is a role in gastroenteritis that was not approved yet [4] . Different reports revealed HBoV prevalence rate in acute gastroenteritis in children. Most of them were dual infection with other viral agents like Rotavirus and Norovirus and Adenovirus [4–6] . No report expressed HBoV gastrointestinal infection in the unique form [9–12] . The most common clinical symptoms of the disease in HBoV positive patients in all the reports all over the world were diarrhea, fever, dehydration, vomiting, and abdominal pain [5,6] .
In acute gastrointestinal infection by HBoV, a study in Pakistan in 2014, HBoV prevalence was 13% that 98% were found to be co-infected with Rotavirus. Amongst the clinical features, fever and vomiting were presenting symptoms in 89% and 87% of children, respectively [9] . The one study in Albania in 2016, shows that HBoV was detected 9.1%. All HBoV-positive patients were co-infected with other enteric viruses (98%) [10] . There was no data about the analysis of the mono-infection of HBoV properties and clinical separates symptoms in these reports.
In a study in Western India in 2017, 5.3% of samples were positive for Human bocavirus, Co-infection of Rotavirus was observed in 21% cases. HBoV infections occurred in children 12 months of age [11] . In other reports in North of India in 2016, HBoV rate was 3% with the Median age of 8 months. All the positive samples had gastrointestinal findings having diarrhea 100%, dehydration 86%, vomiting 70%, fever 62%, and severe abdominal pain 28% [12] . In these reports, there was a consider that HBoV can be a gastrointestinal pathogen, and they analyze this conception in separate parts of the report.
As like as the above hypothesis, In Iran, in Mohammadi study in Tehran, HBoV identified in 14.4% of patients with acute gastroenteritis infection. The main clinical symptoms among HBoV positive patients were diarrhea (83.3%), abdominal pain (81.9%), and vomiting (83.3%) [13] .
An excellent study by De R, et al. In 2017, about the Risk of acute gastroenteritis associated with Human bocavirus infection in children. Among the included 36 studies, the overall HBoV prevalence in acute gastroenteritis cases was 6.90% [14] . This report shows an important confidential chance of HBoV that can be a gastrointestinal pathogen in children.
None of the above reports there weren’t obvious data that confirmed HBoV pathogenic role in acute gastroenteritis in children and infants, and all of them were epidemiological reports. To decision about HBoV pathogenic role in diarrhea heed to many close clinical studies and focus of HBoV Mono infection form of acute gastroenteritis.
Acknowledgments
The researchers thank the children and their parents who participated in the report.
No funding was received for this study.
Conflict of Interest
The authors claim no conflict of interest.
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102(36):12891–12896.
- Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Soderlund-Venermo M. Human bocavirus-the first 5 years. Rev Med Virol. 2012;22(1):46–64.
- Schildgen O. Human bocavirus: lessons learned to date. Pathogens. 2013;2(1):1–12.
- Broccolo F, Falcone V, Esposito S, Toniolo A. Human bocaviruses: Possible etiologic role in respiratory infection. J Clin Virol. 2015;72:75–81.
- Schildgen O, Muller A, Allander T, Mackay IM, Volz S, Kupfer B, et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21(2):291–304.
- Guido M, Tumolo MR, Verri T, Romano A, Serio F, De Giorgi M, et al. Human bocavirus: Current knowledge and future challenges. World J Gastroenterol. 2016;22(39):8684–8697.
- Abdel-Moneim AS, Kamel MM, Hamed DH, Hassan SS, Soliman MS, Al-Quraishy SA, et al. A novel primer set for improved direct gene sequencing of Human bocavirus genotype-1 from clinical samples. J Virol Methods. 2016;228:108–113.
- Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334(7583):35–40.
- Alam MM, Khurshid A, Shaukat S, Sharif S, Suleman RM, Angez M, et al. Human bocavirus in Pakistani children with gastroenteritis. J Med Virol. 2015;87(4):656–663.
- Rosa G La, Libera SD, Iaconelli M, Donia D, Cenko F, Xhelilaj G, et al. Human bocavirus in children with acute gastroenteritis in Albania. J Med Virol. 2016;88(5):906–1010.
- Lasure N, Gopalkrishna V. Molecular epidemiology and clinical severity of Human Bocavirus (HBoV) 1–4 in children with acute gastroenteritis from Pune, Western India. J Med Virol. 2017;89(1):17–23.
- Kapoor R, Dhole TN. Human bocavirus (HBoV1 and HBoV2) in Children with Acute Gastroenteritis from North India. Journal of Antimicrobial Agents. 2016;2(3):1–5.
- Mohammadi M, Armin S, Yazdanpour Z. Human bocavirus infections and co-infections with respiratory syncytial virus and Rotavirus in children with acute respiratory or gastrointestinal disease. Braz J Microbiol. 2020;51(1):45–51.
- De R, Liu L, Qian Y, Zhu R, Deng J, Wang F, et al. Risk of acute gastroenteritis associated with Human bocavirus infection in children: A systematic review and meta-analysis. PLoS One. 2017;12(9):e0184833.
Human bocavirus; Acute gastroenteritis; Infant; Pathogen
Cite this article
Mohammadi M, Sabzi N, Bahrami N. The case report of HBoV acute gastroenteritis in a two-month-old infant. Clin Case Rep J. 2020;1(4):1–3.
© 2020 Mehrdad Mohammadi, This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).
Are you interested in submitting your research paper?
Thank you for choosing us. We recommend you to go through our guidelines before submitting the paper.

Clinical Case Reports Journal is the state-of-the-art platform to showcase medical journals across scientific community and a one-stop solution for publishing the manuscripts of varied medical specialities.
Useful Links
Our contacts.
CLINICAL CASE REPORTS JOURNAL, C/O Infact Publications LLC, 16192 Coastal Highway, Lewes, Delaware 19958 USA
[email protected] https://clinicalcasereportsjournal.com
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Acute gastroenteritis in children
Elizabeth jane elliott.
- Author information
- Article notes
- Copyright and License information
Correspondence to: E Elliott, c/o Children's Hospital at Westmead, Westmead 2145, NSW, Australia [email protected]
Corresponding author.
Accepted 2006 Nov 9.
Acute gastroenteritis accounts for millions of deaths each year in young children, mostly in developing communities. In developed countries it is a common reason for presentation to general practice or emergency departments and for admission to hospital. Dehydration, which may be associated with electrolyte disturbance and metabolic acidosis, is the most frequent and dangerous complication. Optimal management with oral or intravenous fluids minimises the risk of dehydration and its adverse outcomes. Routine use of antibiotics, antidiarrhoeal agents, and antiemetics is not recommended and may cause harm. Prevention is the key to controlling gastroenteritis, and recently licensed, highly effective rotavirus vaccines will have a major effect on public health.
Sources and selection criteria
I searched the Cochrane Library database using the keywords “acute gastroenteritis” (all text), “acute disease”, “gastroenteritis”, and “child”. I searched Medline via PubMed clinical queries using the keywords “gastroenteritis” together with “oral rehydration”, “antidiarrheal”, “antiemetic”, “probiotic”, and “zinc” with the options “find systematic reviews” and “search by study category—therapy.” The options of “aetiology” and “diagnosis” were also applied using the term “gastroenteritis”. I also searched the child health section of Clinical Evidence and reviewed the reference lists of publications found during searches for other relevant manuscripts

What is the epidemiology and impact of gastroenteritis?
Acute gastroenteritis—diarrhoea or vomiting (or both) of more than seven days duration—may be accompanied by fever, abdominal pain, and anorexia. Diarrhoea is the passage of excessively liquid or frequent stools with increased water content. Patterns of stooling vary widely in young children, and diarrhoea represents a change from the norm. 1 Worldwide, 3-5 billion cases of acute gastroenteritis and nearly 2 million deaths occur each year in children under 5 years. 2 In the United States, gastroenteritis accounts for about ∼10% (220 000) of admissions to hospital, more than 1.5 million outpatient visits, and around 300 deaths in children under 5 annually, with a cost of around $1bn (£0.5bn; €0.8bn). 2 In the same age group in Australia, about 10 000 hospital admissions, 22 000 visits to emergency departments, and 115 000 general practice consultations occur annually for rotavirus alone, with an estimated cost of $A30m (£12m; €18m; $23m). 3 In the United Kingdom, 204 of 1000 consultations with general practitioners in children under 5 are for gastroenteritis, and the annual hospital admission rate in this group is about seven per 1000 children. 4 Children in childcare settings are often infected but asymptomatic and may unwittingly transmit infection.
Children with poor nutrition are at increased risk of complications. In the north end of Australia, Aboriginal and Torres Strait Islander children have increased rates of admission for gastroenteritis, malnutrition, comorbidity, and electrolyte disturbance (especially hypokalaemia) and a longer hospital stay than their non-indigenous counterparts. 5 The cost of gastroenteritis to the community is huge but often underestimated if costs to the family, including lost time at work, are not considered.
Summary points
Rotavirus is the most common cause of acute gastroenteritis worldwide and vaccination will have a major impact on disease rates, morbidity, and mortality
Most children are not dehydrated and can be managed at home
Dehydration, metabolic acidosis, and electrolyte disturbance can be prevented and treated by fluid therapy
Most children with mild-moderate dehydration can be treated with oral or enteral rehydration using low osmolality oral rehydration solutions
Severely dehydrated or shocked children usually need intravenous fluids and hospital admission
Drugs are usually unnecessary and may do harm
General practitioners have an important role in prevention, through encouraging breastfeeding, recommending and advocating free access to rotavirus vaccination, and educating carers about personal and food hygiene
What are the causes and clinical characteristics?
Box 1 lists some causes of acute gastroenteritis in children. Worldwide, most cases are due to viral infection (fig 1; box 2), with rotaviruses and noroviruses being most common. Viral infections damage small bowel enterocytes and cause low grade fever and watery diarrhoea without blood. Rotavirus infection is seasonal in temperate climates, peaking in late winter, but occurs throughout the year in the tropics. Rotavirus strains vary by season and geographically within countries. 6 The peak age for infection is between 6 months and 2 years, and the mode of spread is by the faecal-oral or respiratory route.
Fig 1 Rotavirus particles seen under the electron microscope. Courtesy of Alan Philips
Box 1 Causes of acute gastroenteritis in children
Viruses (∼70%).
Rotaviruses
Noroviruses (Norwalk-like viruses)
Enteric adenoviruses
Caliciviruses
Astroviruses
Enteroviruses
Protozoa (<10%)
Cryptosporidium
Giardia lamblia
Entamoeba histolytica
Bacteria (10-20%)
Campylobacter jejuni
Non-typhoid Salmonella spp
Enteropathogenic Escherichia coli
Shigella spp
Yersinia enterocolitica
Shiga toxin producing E coli
Salmonella typhi and S paratyphi
Vibrio cholerae
Strongyloides stercoralis
Box 2 Fictitious clinical case
Presentation.
Tina is a 6 month old bottle fed infant, brought to your general practice surgery with a 12 hour history of diarrhoea (10 watery green stools without blood) and vomiting (eight yellow non-bilious vomits). Her mother is worried because she is irritable with fever (38°C). Her brother, who attends nursery, has just got over a bout of “gastro.” She is an active baby with perianal excoriation and a weight of 5.5 kg. She has a sunken fontanelle and decreased skin turgor (pinch test 3 sec). She passes urine during the examination. On review of her health record you note she has lost 0.5 kg since last weighed two weeks ago. You diagnose viral gastroenteritis and refer the child to the local paediatric unit.
Rotavirus infection is confirmed by commercial enzyme linked immunosorbent assay and she is assessed as having moderate (8%) dehydration (on the basis of recent weight loss, decreased skin turgor, raised serum urea, and mild metabolic acidosis).
The child is reluctant to drink so you give an oral rehydration solution by nasogastric tube to rehydrate her over six hours in the emergency department. She is observed overnight to ensure that she is drinking an adequate amount and passing urine.
Bacterial pathogens such as C ampylobacter jejuni and Salmonella spp invade the lining of the small and large intestine and trigger inflammation. 7 Children with bacterial gastroenteritis are more likely to have high fever and may have blood and white blood cells in the stool. Bacterial pathogens occasionally spread systemically, especially in young children. Infection with Shiga toxin producing Escherichia coli or Shigella dysenteriae may cause haemorrhagic colitis (with severe bloody diarrhoea), which may be complicated by haemolytic uraemic syndrome. This syndrome is endemic worldwide and characterised by acute onset of microangiopathic haemolytic anaemia (fig 2), thrombocytopenia, acute renal impairment, and multisystem involvement (see appendix on bmj.com). 8
Fig 2 Typical peripheral blood film in a patient with haemolytic uraemic syndrome, microangiopathic haemolytic anaemia, and thrombocytopenia. It shows irregular, fragmented, and helmet shaped red blood cells (schistocytes) and an immature platelet
The enteric fevers (due to Salmonella typhi and S paratyphi ) cause severe illness in young children, characterised by high swinging fever, diarrhoea or constipation, leucopenia, and sometimes central nervous system involvement, including encephalopathy. 7 Encephalopathy is a rare complication of non-typhoid Salmonella infection. Vibrio cholera toxin causes chloride and water secretion from the small bowel but does not damage the intestinal mucosa; it results in “rice water” stools that have a high sodium content but do not contain blood or white blood cells. 7
Gastroenteritis is acquired by person to person spread or ingestion of contaminated food and drink (“food poisoning”). 7 Undercooked, or inappropriately stored cooked or processed meats (chicken, beef, pork) and seafood are common sources of bacterial pathogens. Ingestion of food containing toxins produced by bacterial contaminants (for example, Staphylococcus aureus in ice cream or Bacillus cerus in reheated rice) causes rapid onset of vomiting or diarrhoea (or both). Water may be contaminated with bacteria, viruses, or protozoa including Giardia lamblia , cryptosporidium, V cholera , and Entamoeba histolytica, which causes amoebic dysentery. With increasing rates of overseas travel and immigration, clinicians in developed countries increasingly see children with “traveller's diarrhoea” caused by a range of organisms not normally seen in that environment.
Unanswered research questions in acute gastroenteritis
How safe and effective is home based care for children with mild-moderate dehydration?
What role do food based oral rehydration solutions have in developed communities?
What is the role and safety of new generation antiemetics and antidiarrhoeal agents?
What is the role of zinc supplementation in well nourished children?
Do probiotics have a role as adjuvant therapy, and what type, dose, and regimen is optimal?
How is it diagnosed?
Diagnosis can be made clinically. Information should be sought about recent contact with people with gastroenteritis, nature and frequency of stool and vomitus, fluid intake and urine output, travel, and use of antibiotics and other drugs that may cause diarrhoea. Chronic constipation is common in children, and faecal overflow incontinence may present as spurious diarrhoea. Diarrhoea and vomiting are non-specific symptoms in young children, and the diagnosis of gastroenteritis should be questioned in children with high fever, prolonged symptoms, or signs suggesting a surgical cause (such as severe abdominal pain, bilious vomiting, abdominal mass). Children with diabetes mellitus and inborn errors of metabolism may present with vomiting. Children with underlying diseases may be at increased risk of complications and referral to a paediatric service should be considered.
It is not necessary or practical to take stool specimens from all children with gastroenteritis. Samples should be taken during outbreaks—especially in childcare, school, hospital, or residential settings—where there is a public health imperative to identify the pathogen and establish its source. Samples should be cultured for bacteria and tested for viral pathogens. Testing for rotavirus, norovirus, and sometimes other viruses is available in most children's hospitals using methods for rapid antigen detection (such as enzyme linked immunosorbent assay). Rapid diagnosis allows for isolation of the child to prevent nosocomial infection, which is common and is often used as a marker of the effectiveness of precautions to control contact infection. Stool samples should also be taken from children with bloody diarrhoea, a history of recent foreign travel, and from young or immunocompromised children with high fever. In many countries legislation requires clinicians to notify public health authorities about a range of viral and bacterial infections.
How is dehydration assessed?
It is important to assess hydration in gastroenteritis as hydration status determines the immediate management of this condition. The infant or child with profuse watery diarrhoea and frequent vomiting is most at risk. Clinicians often overestimate the extent of dehydration. Clinical signs are usually not present until a child has lost at least 5% of his or her body weight. Documented recent weight lost is a good indicator of the degree of dehydration, but this information is rarely available. The best clinical indicators of more than 5% dehydration are prolonged capillary refill, abnormal skin turgor, and absent tears. 9 The recommendations for assessing and managing dehydration shown in table 1 are adapted from the World Health Organization classification and are supported by the literature. 9 10 11 Serum electrolytes are not routinely required but should be measured before and after starting intravenous fluids.
Assessment and management of dehydration
*A measure of skin turgor. Assessed by pinching the skin of the abdomen or thigh between the thumb and the bent forefinger in a longitudinal manner. Results are unreliable in obese or severely malnourished children.
†Other signs of severe dehydration include circulatory collapse (weak rapid pulse, cool or blue extremities, prolonged capillary refill time, or hypotension), rapid breathing, and sunken anterior fontanelle.
How is gastroenteritis treated?
Table 1 summarises the management of dehydration 2 4 10 11 12 13 14 15 16 17 18 19 20 21 22 23 and table 2 lists the type of evidence supporting management decisions in gastroenteritis (a longer version of table 2 (table A) is available on bmj.com). Management aims to prevent and treat dehydration, maintain nutrition, and minimise harm.
Evidence based management of gastroenteritis
Which fluid therapy?
Children with no dehydration or mild dehydration can usually be managed at home, although children with high risk for complications or who cannot be adequately cared for at home should be considered for admission. 2 11 13 Children with mild-moderate dehydration who do not tolerate oral fluids should be admitted for observation. Oral rehydration solutions are preferable to other clear fluids for preventing and treating dehydration. 2 4 11 Fluids high in sugar (such as cola, apple juice, and sports drinks, which contain ≤20 mmol/l sodium and have a high osmolality of 350-750 mOsm/l) may exacerbate diarrhoea and should be avoided. 11 Breast feeding should be continued during acute gastroenteritis and supplemented with an oral rehydration solution if needed. 11 12
Although most children with dehydration drink readily, some refuse rehydration solutions because they dislike the taste, feel nauseated, or have profuse vomiting. Older children may be afraid of vomiting and parents may perceive fluids are the cause of vomiting. If small sips cannot be tolerated, use of a syringe can help in infants. If oral intake is inadequate, a fine bore nasogastric tube is usually well tolerated. 14 15 Alternatively, fluids may be given intravenously. 11 Enteral (oral or nasogastric) and intravenous fluids are equally safe and effective for mild-moderate dehydration, 14 15 and rehydration can usually be achieved in four to six hours.
In developed communities children with severe dehydration are routinely admitted for intravenous therapy, 2 11 although enteral rehydration has been used safely in severe dehydration with fewer adverse effects than intravenous therapy (table 2). 14 15 Children with shock require intravenous resuscitation before rehydration. 2 10 11
The most common adverse effect of intravenous cannulation is infiltration at the cannula site, but infection, pain, bleeding, and physical and emotional trauma may also occur. Intravenous therapy is more expensive than oral rehydration therapy and requires hospital admission. Iatrogenic complications—especially electrolyte disturbance due to inappropriate composition, rate of administration, or volume of intravenous fluids—may lead to complications, including hyponatraemia with brain injury or death (box 3). If rapid intravenous rehydration is used, careful supervision is needed to avoid fluid overload (dehydration is often overestimated) and electrolyte imbalance.
Box 3 Complications of acute gastroenteritis
Dehydration
Metabolic acidosis
Electrolyte disturbance (hypernatraemia, hyponatraemia, hypokalaemia)
Carbohydrate (lactose, glucose) intolerance
Susceptibility to reinfection
Development of food (cow's milk, soy protein) intolerance
Haemolytic uraemic syndrome
Iatrogenic complications (due to inappropriate composition or amount of intravenous fluids)
Which oral rehydration solution?
Solutions with low osmolality (200-250 mOsm/l) and sodium (60-70 mmol/l) that contain glucose, potassium, and a base (such as citrate) are recommended for developed and developing communities (table 2; table B on bmj.com). 16 17 18 Although cereal based oral dehydration solutions are beneficial in cholera-like diarrhoea, 19 evidence of benefit in non-cholera diarrhoea is scant and further trials are needed to evaluate efficacy and cost effectiveness.
What about diet?
In a systematic review, probiotics—used as an adjunct to oral rehydration therapy—decreased the duration of diarrhoea, especially in rotavirus gastroenteritis (table 2). 20 Further research is needed to determine the optimal type, dosage, and regimen of probiotics before they are recommended for routine use.
Additional educational resources
Clinical resources.
Clinical evidence ( www.clinicalevidence.com/ceweb/conditions/chd/chd.jsp )
Cochrane Library (www.cochrane.org)
Evidence-based Paediatrics and Child Health ( www.evidencebasedpediatrics.com )
Managing acute gastroenteritis among children ( www.cdc.gov/mmwr/preview/mmwrhtml/rr5216al.htm )
Glass RI, Parashar UD. The promise of new rotavirus vaccines. N Engl J Med 2006;354:75-7
Information resources for patients
BUPA. Gastroenteritis in children (http://hcd2.bupa.co.uk/fact_sheets/html/gastroenteritis_children.html)
Cincinnati Children's Hospital Medical Centre, USA. Gastroenteritis (www.cincinnatichildrens.org/health/info/abdomen/diagnose/gastroenteritis.htm)
Health Institute. An Australian government initiative. Gastroenteritis in children (www.healthinsite.gov.au/topics/Gastroenteritis_in_Children)
Children should resume their normal diet once their appetite returns. 2 10 11 Published guidelines recommend early reintroduction of milk and solids including complex carbohydrates, lean meats, yogurt, and vegetables, but foods high in fat and sugars should be avoided. 3 Early refeeding reduces the duration of diarrhoea. In formula fed infants feeds do not need to be diluted when reintroduced. 21
What is the role of drugs?
Drugs are rarely needed. 3 10 11 They deal with the symptoms rather than causes of disease and may distract from the use of appropriate fluid therapy. Antibiotics are not indicated in viral or uncomplicated bacterial gastroenteritis and may cause harm. For example, in non-typhoid Salmonella infections antibiotics increase the risk of prolonged carriage and disease relapse. Treating gastroenteritis due to Shiga toxin producing E coli with antibiotics may increase the risk of haemolytic uraemic syndrome. Antibiotics are required, however, for bacterial gastroenteritis complicated by septicaemia and in cholera, shigellosis, amoebiasis, giardiasis, and enteric fever.
Antidiarrhoeal and antiemetic agents are not recommended for routine use because of the risk of adverse effects (table 2; table A on bmj.com). 3 10 11 Although new generation antiemetics (such as the serotonin antagonist ondansetron) do not have extrapyramidal effects and reduce the duration and frequency of vomiting, they also increase diarrhoea. Antimotility agents (such as loperamide) decrease the duration of diarrhoea, but they have potential severe adverse effects and evidence that benefits outweigh potential harms is lacking. 6
In developing countries, oral zinc given at the onset of symptoms decreases the duration and severity of acute diarrhoea and is recommended by the WHO. 10 Vitamin A does not influence the course of acute gastroenteritis.
Is a lactose-free diet necessary?
Carbohydrate (particularly lactose) intolerance is a common complication of viral gastroenteritis as a result of damage to and loss of mature enterocytes containing lactase. Lactose intolerance is usually mild and self limiting and does not require treatment. 3 21 If lactose intolerance persists, a lactose-free formula is recommended for four to six weeks. 3 21 The damaged gut is more permeable to foreign antigens and intolerance to food proteins (β lactoglobulin in cow's milk and other proteins) is occasionally seen after gastroenteritis; it can be managed by a period of dietary exclusion. 3 10 11
Can gastroenteritis be prevented?
Although rotavirus may be spread in aerosols, gastroenteritis is usually spread by the faecal-oral route. Bacterial gastroenteritis can occur in young children served uncooked fermented meats, undercooked hamburgers, unwashed fruits and salads, and water contaminated by animal faeces. Gastroenteritis may also be acquired from environmental sources, such as children's animal farms, swimming pools, and beaches. Good hygiene is important to prevent spread of infection. This includes careful hand washing, nappy disposal, and preparation and storage of food and drinking water, as outlined in the WHO's five step guide to safe food (table C on bmj.com). Hygiene is particularly important in institutions, including hospitals where nosocomial infection is common.
Tips for non-specialists
Differential diagnoses.
Other infections, such as urinary tract infection, otitis media, pneumonia, septicaemia
Surgical causes, such as intussusception, appendicitis, small intestinal obstruction (including malrotation)
Taking antibiotics or other drugs
Spurious diarrhoea; for example, in chronic constipation with overflow incontinence
Non-infectious diseases such as diabetic ketoacidosis, inborn errors of metabolism
Occasionally acute infectious gastroenteritis unmasks gastrointestinal disease (such as coeliac disease, chronic inflammatory bowel disease), so if diarrhoea persists beyond two weeks take a family and medical history and do appropriate investigations
When to refer to paediatric service
If diagnosis in doubt
Gastroenteritis in a young infant (<6 months)
High risk of dehydration—worsening diarrhoea and vomiting with significant fluid loss
Severe dehydration or shock
Severe abdominal pain, localised tenderness, or mass
Evidence of anaemia, thrombocytopenia, poor urine output, or hypertension (think haemolytic uraemic syndrome)
Increased risk of complications—underlying disease (such as diabetes), malnutrition, renal failure, high fever
Parent or carer unable to manage the child at home
Persistent diarrhoea beyond two weeks may indicate complications such as reinfection, lactose intolerance, or underlying bowel disease
A major recent advance in prevention has been the development and licensing of two oral rotavirus vaccines, whose safety and efficacy have been confirmed in recent large scale trials, each involving more than 60 000 children. 24 25 Rotateq (Merck) is a three dose live human-bovine pentavalent reassortant vaccine. Rotarix (GSK) is two dose attenuated human (strain G1P) monovalent vaccine. Both vaccines are highly immunogenic. They provide cross protection against common serotypes and decrease rates of severe gastroenteritis, the need for intravenous fluids, and hospital admission. Importantly, neither is associated with appreciable adverse effects or increased risk of intussusception, which was seen with the first licensed vaccine, RotaShield. Free access to rotavirus vaccine in all communities is imperative and will have an enormous impact on childhood morbidity and mortality.
Supplementary Material
I am grateful to Michael Fasher and Alison Kesson for useful feedback on this article before submission.
Competing interests: None declared.
Tables A-C and an appendix are on bmj.com
- 1. Tham EB, Nathan R, Davidson GP, Moore DJ. Bowel habits of healthy Australian children aged 0-2 years. J Paediatr Child Health 1996;32:504-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. King CK, Glass R, Bresee JS, Duggan C; Centers for Disease Control and Prevention. Managing acute gastroenteritis among children. MMWR Recomm Rep 2003;52(RR16):1-16. [ Google Scholar ]
- 3. Galati JC, Harsley S, Richmond P, Carlin JB. The burden of rotavirus-related illness among young children on the Australian health care system. Aust N Z J Public Health 2006;30:416-21. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Elliott E, Dalby-Payne J. Gastroenteritis in children. Clin Evid 2006;15:1-7.16973003 [ Google Scholar ]
- 5. Ruben AR, Fisher DA. The casemix system of hospital funding can further disadvantage Aboriginal children. Med J Aust 1998;169(8 suppl):S6-10. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Kirkwood CD, Bogdanovic-Sakran N, Cannan D, Bishop RF, Barnes GL. National rotavirus surveillance program annual report 2004-5. Commun Dis Intell 2006;30:133-6. [ PubMed ] [ Google Scholar ]
- 7. US Food and Drug Administration Center for Food Safety and Applied Nutrition. Bacterial explanations. Bacterial testing and analysis at FPL. www.fplabs.com/bactlist.htm.
- 8. Elliott E, Robins-Browne R. Hemolytic uremic syndrome. In: Moyer VA, ed. Problems in child and adolescent medicine. USA: Elsevier, 2005;35:310-30. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? The rational clinical examination. JAMA 2004;291:2746-54. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. World Health Organization. The treatment of diarrhoea—a manual for physicians and other senior health workers. 4th rev. Geneva: WHO, 2005.
- 11. Acute Gastroenteritis Guideline Team. Cincinnati Children's Hospital Medical Center. Evidence-based care guidelines. Gastroenteritis. 2005. www.cincinnatichildrens.org/svc/alpha/h/health-policy/ev-based/gastro.htm.
- 12. Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001;32:331-51. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. McConnochie KM, Conners GP, Lu E, Wilson C. How commonly are children hospitalized for dehydration eligible for care in alternative settings? Arch Pediatr Adolesc Med 1999;153:1233-41. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Fonseca BK, Holdgate A, Craig JC. Enteral vs intravenous rehydration therapy for children with gastroenteritis: a meta-analysis of randomized controlled trials. Arch Pediatr Adolesc Med 2004;158:483-90. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev 2006;(3):CD004390. [ DOI ] [ PMC free article ] [ PubMed ]
- 16. Hahn S, Kim Y, Garner P. Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children. Cochrane Database Syst Rev 2002;(1):CD002847. [ DOI ] [ PubMed ]
- 17. Murphy C, Hahn S, Volmink J. Reduced osmolarity oral rehydration solution for treating cholera. Cochrane Database Syst Rev 2004;(4):CD003754. [ DOI ] [ PubMed ]
- 18. WHO/Unicef. Joint statement. Oral rehydration salts (ORS)—a new reduced osmolarity formulation. www.who.int/medicines/publications/pharmacopoeia/OralRehySalts.pdf.
- 19. Gore SM, Fontaine O, Pierce NF. Impact of rice based oral rehydration solution on stool output and duration of diarrhoea: meta-analysis of 13 clinical trials. BMJ 1992;304:287-91. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev 2003;(4):CD003048. [ DOI ] [ PubMed ]
- 21. Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics 1994;93:17-27. [ PubMed ] [ Google Scholar ]
- 22. Elliott EJ, Peadon E. Expert commentary on Alhashimi D, Alhashimi H, Fedorowicz Z. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev Evidence-Based Child Health 2006;(3):CD005506. [ DOI ] [ PubMed ]
- 23. Bahl R, Baqui A, Bhan MK, Bhatnagar S, Black RE, Brooks A, et al. Effect of zinc supplementation on clinical course of acute diarrhoea. Report of a meeting, New Delhi, 7-8 May 2001. J Health Popul Nutr 2001;19:338-46. [ PubMed ] [ Google Scholar ]
- 24. Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriquez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006;354:23-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006,354:11-22. [ DOI ] [ PubMed ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (256.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- State-of-the-Art Reviews
- Voices of ID
- Author Guidelines
- Open Access
- Why Publish
- IDSA Journals Calls for Papers
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Supplementary data.
- < Previous
Incidence, Etiology, and Severity of Acute Gastroenteritis Among Prospectively Enrolled Patients in 4 Veterans Affairs Hospitals and Outpatient Centers, 2016–2018
- Article contents
- Figures & tables
Cristina V Cardemil, Neha Balachandran, Anita Kambhampati, Scott Grytdal, Rebecca M Dahl, Maria C Rodriguez-Barradas, Blanca Vargas, David O Beenhouwer, Karen V Evangelista, Vincent C Marconi, Kathryn L Meagley, Sheldon T Brown, Adrienne Perea, Cynthia Lucero-Obusan, Mark Holodniy, Hannah Browne, Rashi Gautam, Michael D Bowen, Jan Vinjé, Umesh D Parashar, Aron J Hall, Incidence, Etiology, and Severity of Acute Gastroenteritis Among Prospectively Enrolled Patients in 4 Veterans Affairs Hospitals and Outpatient Centers, 2016–2018, Clinical Infectious Diseases , Volume 73, Issue 9, 1 November 2021, Pages e2729–e2738, https://doi.org/10.1093/cid/ciaa806
- Permissions Icon Permissions
Acute gastroenteritis (AGE) burden, etiology, and severity in adults is not well characterized. We implemented a multisite AGE surveillance platform in 4 Veterans Affairs Medical Centers (Atlanta, Georgia; Bronx, New York; Houston, Texas; and Los Angeles, California), collectively serving >320 000 patients annually.
From 1 July 2016 to 30 June 2018, we actively identified inpatient AGE case patients and non-AGE inpatient controls through prospective screening of admitted patients and passively identified outpatients with AGE through stool samples submitted for clinical diagnostics. We abstracted medical charts and tested stool samples for 22 pathogens by means of multiplex gastrointestinal polymerase chain reaction panel followed by genotyping of norovirus- and rotavirus-positive samples. We determined pathogen-specific prevalence, incidence, and modified Vesikari severity scores.
We enrolled 724 inpatients with AGE, 394 non-AGE inpatient controls, and 506 outpatients with AGE. Clostridioides difficile and norovirus were most frequently detected among inpatients (for AGE case patients vs controls: C. difficile , 18.8% vs 8.4%; norovirus, 5.1% vs 1.5%; P < .01 for both) and outpatients (norovirus, 10.7%; C. difficile , 10.5%). The incidence per 100 000 population was highest among outpatients (AGE, 2715; C. difficile, 285; norovirus, 291) and inpatients ≥65 years old (AGE, 459; C. difficile, 91; norovirus, 26). Clinical severity scores were highest for inpatient norovirus, rotavirus, and Shigella /enteroinvasive Escherichia coli cases. Overall, 12% of inpatients with AGE had intensive care unit stays, and 2% died; 3 deaths were associated with C. difficile and 1 with norovirus. C. difficile and norovirus were detected year-round with a fall/winter predominance.
C. difficile and norovirus were leading AGE pathogens in outpatient and hospitalized US veterans, resulting in severe disease. Clinicians should remain vigilant for bacterial and viral causes of AGE year-round.
Acute gastroenteritis (AGE), characterized by diarrhea, vomiting, fever, and abdominal pain, causes 1.3 million deaths globally every year [ 1 ]. In the United States, AGE causes 179 million cases and 1 million hospitalizations annually [ 2 , 3 ]. Historically, AGE prevention and control efforts have focused on children, but adults experience a substantial proportion of illness; most US deaths are in older adults [ 3 , 4 ]. Both viral and bacterial pathogens play important roles in AGE across all age groups, and norovirus and Clostridioides difficile are important emerging infectious causes of AGE among US adults, including AGE-associated deaths [ 4 ]. In the United States, norovirus causes 20 million AGE illnesses and about 800 deaths every year [ 5 ]. C. difficile, increasingly observed in community and acute care settings, caused an estimated 453 000 infections with 29 000 associated deaths in 2011 [ 6 ]. Other pathogens, such as rotavirus, contribute to AGE in children [ 7 ], but the adult burden is largely unknown.
Despite accumulating evidence on the importance of specific pathogens for AGE, population-based studies of the endemic burden of all-cause AGE in adults are rare. Although some data are available on bacterial pathogens [ 8 ], lack of population-based data on viral pathogens hinders estimations of pathogen-specific prevalence and incidence to inform clinical management and interventions, such as vaccines. Past viral AGE burden studies used indirect methods, such as billing codes without laboratory confirmation of disease; relied on clinician-ordered diagnostic testing results, which can bias detection away from viral pathogens; or focused on groups or populations that limit generalizability [ 3 , 7 , 9–11 ]. Moreover, traditional surveillance methods have been limited by infrequent diagnostic testing, except in severe AGE cases or outbreak settings [ 12 ]. With norovirus and C. difficile vaccines in clinical trials among all age groups [ 13 , 14 ], robust pathogen-specific burden estimates are needed for baseline data that can serve as benchmarks for future impact evaluations.
To address these gaps, SUPERNOVA (Surveillance Platform for Enteric and Respiratory Infectious Organisms in the Veterans Affairs population) was established as a multisite outpatient and inpatient surveillance platform in VA medical centers (VAMCs) in Atlanta, Georgia; Bronx, New York; Houston, Texas; and Los Angeles, California. The project was initially designed to estimate the norovirus burden through passive surveillance [ 15 , 16 ]; in 2015, an active surveillance pilot was launched at 1 site to characterize the AGE etiologic burden [ 17 ]. Subsequently, SUPERNOVA expanded to include active and passive AGE surveillance at all 4 sites, as well as testing for 22 gastrointestinal pathogens.
In the current article, we describe findings from the first 2 years of prospective, inpatient and outpatient multisite surveillance for AGE, with standardized methods and laboratory testing. We sought to estimate prevalence and incidence of outpatient encounters and inpatient hospitalizations for AGE due to norovirus, in the context of other viral, bacterial, and parasitic pathogens. Secondary objectives included describing the severity of illness among hospitalized patients with AGE caused by specific pathogens. Findings will inform clinical AGE management in adults and future treatment and control interventions for AGE pathogens.
Participants and Case Definitions
SUPERNOVA sites served 322 468 unique veterans in outpatient and inpatient settings in fiscal year 2018 (24 485 in the Bronx, 83 477 in Los Angeles, 104 926 in Houston, and 109 580 in Atlanta). For inpatient active surveillance, veterans ≥18 years of age admitted to a participating VAMC were screened for eligibility during 2 surveillance years (from 1 July 2016 to 30 June 2017 and from 1 July 2017 to 30 June 2018). Active cases were defined as the occurrence of ≥3 loose stools, ≥2 episodes of vomiting, or ≥1 episode of both loose stool and vomiting in a 24-hour period and illness ≤10 days in duration. Patients were excluded if symptoms were present for >10 days or attributed to a noninfectious cause; if they were transferred from another hospital after admission of >48 hours; or if they were enrolled within the previous month. Controls were patients without AGE symptoms for ≥2 weeks, and were frequency matched to case patients by age bracket (<65 or ≥65 years) and admission date (±1 week). All inpatients meeting inclusion criteria during core surveillance hours were invited to participate ( Supplementary Methods ). For outpatient passive surveillance, patients with stool specimens collected for clinician-requested diagnostic testing in each site’s catchment area that met the case definition—acute onset of AGE symptoms recorded in the medical chart—were included in outpatient surveillance.
Laboratory Testing
Whole stool specimens were tested at each VAMC using the BioFire FilmArray Gastrointestinal Panel (bioMérieux), a polymerase chain reaction (PCR)–based multidiagnostic assay with targets for 22 viral, bacterial, and parasitic pathogens ( https://www.biomerieux-diagnostics.com/filmarrayr-gi-panel ). Stool samples with norovirus or rotavirus detected using this panel were shipped to the Centers for Disease Control and Prevention for real-time reverse-transcription quantitative PCR testing and genotyped by sequencing [ 18–21 ].
Statistical Analysis
The final analysis included the Houston VAMC from 1 July 2016 through 30 November 2016, and all 4 sites after the Atlanta, Bronx, and Los Angeles VAMCs began surveillance from 1 December 2016 through 30 June 2018. We calculated the prevalence of each pathogen among case patients and controls; examined the severity of illness, including duration of illness, hospital stay, modified Vesikari score ( Supplementary Table 1 ), and intensive care unit (ICU) stay; and identified deaths by follow-up interview for inpatients, stratified by pathogens. We generated confidence intervals by bootstrapping or by assuming Poisson and conducted comparisons with Mann-Whitney, χ 2 , and Fisher exact tests in SAS 9.4 software (SAS Institute). We report in-depth results for case patients where pathogen prevalence significantly differed between case patients and controls.
The incidence per 100 000 person-years among a VA patient population was calculated based on previously published methods [ 15 , 17 ]. To identify only incident episodes of AGE, we counted encounters within 30 days as a single episode. Inpatient incidence estimates were stratified as community onset (illness onset before admission or on the day of or the day after admission) or hospital onset (illness onset ≥2 days after admission).
Enrollment, Demographics, and Risk Factors for AGE Case Patients
Over the 2-year surveillance period, 793 inpatients were enrolled (53% of eligible patients), and 724 (91%) completed all baseline study procedures ( Figure 1 ). For outpatients, 544 stool specimens were available from clinician-ordered diagnostic testing; 506 (93%) met eligibility criteria and were included in analyses ( Figure 1 ). Stool specimens were collected a median of 3 days after symptom onset for inpatients, and 4 days after onset for outpatients.

Screening, enrollment and study completion for inpatients with acute gastroenteritis (AGE), inpatient controls, and outpatients with AGE, from July 2016 to June 2018.
Most participants were male, reflective of the veteran population. Their median ages were 64 years for inpatients with AGE and 63 years for controls ( P = .5) ( Table 1 ). Inpatients with AGE were more likely than controls to have human immunodeficiency virus (HIV) (8.4% vs 2.4%; P = .003), severe renal disease (18.1% vs 4.9%; P < .001), or household contact with a patient with AGE (5.2% vs 2.3%; P = .02) or to be transplant recipients (2.8% vs 0%; P = .01).
Demographic Characteristics and Risk Factors for Inpatients with Acute Gastroenteritis (AGE), Inpatient Controls, and Outpatients With AGE, July 2016 to June 2018
Abbreviations: AGE, acute gastroenteritis; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; VA, Veterans Affairs.
a Data represent no. (%) of patients and controls unless otherwise specified. Some data were missing for race, ethnicity, residence before admission, previous hospitalization, prior antibiotic use, and household contact, representing <5% for each variable.
b P values based on χ 2 , Fisher exact, or Wilcoxon rank-sum tests used to compare inpatients with AGE and controls.
c “Other” includes American Indian/Alaskan Native, Native Hawaiian/Pacific Islander, multiple races, or other race.
d Non-VA sources of care included a physician’s office, non-VA emergency room, urgent care center, or other non-VA site.
e Data were available for HIV/AIDS, cancer, transplants, and immunosuppressive therapy for case patients (n = 667) from October 2016 to June 2018 and for controls (n = 206) from October 2017 to June 2018. Sensitivity analysis of data from only October 2017 to June 2018 resulted in similar proportions and levels of significance. For severe renal disease (severe kidney disease or kidney failure (stage 4 [glomerular filtration rate 15–29] or 5 [glomerular filtration rate <15, or requirement for dialysis]) and prior hospitalization within 30 days, data were available for case patients (n = 331) and controls (n = 206) from October 2017 to June 2018.
Pathogen Prevalence, Seasonality, and Laboratory Testing
Among inpatients, prevalence of 6 pathogens differed significantly between case patients and controls: C. difficile (18.8% vs 8.4%; P < .001), norovirus (5.1% vs 1.5%; P = .003), Campylobacter (3.9% vs 0.5%; P <.001), Shigella /enteroinvasive Escherichia coli (EIEC) (2.2% vs 0%; P = .003), Salmonella (1.8% vs 0.3%; P = .03), and rotavirus (1.5% vs 0%; P = .01) ( Figure 2A ). In the outpatient setting, norovirus (10.7%) and C. difficile (10.5%) were leading pathogens ( Figure 2B ). Among both inpatients and outpatients, most patients with C. difficile or norovirus (inpatients, 84% and 70%, respectively; outpatients, 75% and 80%) had only a single pathogen detected with FilmArray; the most common codetection was C. difficile and norovirus (in 8 inpatients and 2 outpatients). The highest norovirus prevalence was in adults aged ≥85 years (13%–36% by setting; Supplementary Table 2 ).

Prevalence of pathogens detected by means of the BioFire FilmArray Gastrointestinal Panel, from July 2016 to June 2018. A, Inpatients with acute gastroenteritis (AGE) and non-AGE inpatient controls. *Significant difference ( P < .05; χ 2 test) in prevalence between case patients and controls for the following pathogens: Clostridioides difficile ( P < .001), norovirus ( P = .003), Campylobacter spp. ( P < .001), Shigella /enteroinvasive Escherichia coli (EIEC) ( P = .003), Salmonella ( P = .03), and rotavirus ( P = .01). The following pathogens are on the FilmArray panel but are not included because there were no positive detections: Vibrio cholerae , Escherichia coli O157, Aeromonas , and Cyclospora cayetanensis . Two stool specimens from inpatient controls were excluded owing to inconclusive results. B, Outpatients with AGE. The following pathogens on the FilmArray panel are not included because there were no positive detections: Entamoeba histolytica, Plesiomonas shigelloides, V. cholerae, E. coli O157, and Aeromonas. Two outpatient stool specimens were excluded owing to inconclusive results. Abbreviations: EAEC, enteroaggregative Escherichia coli ; EPEC, enteropathogenic Escherichia coli ; ETEC, enterotoxigenic Escherichia coli ; G. lamblia, Giardia lamblia ; STEC, Shiga-like toxin-producing Escherichia coli ; Y. enterocolitica, Yersinia enterocolitica .
Most AGE cases in inpatients were community onset (80%) rather than hospital onset (20%); 78% of C. difficile cases, and 95% of norovirus cases were community onset ( Supplementary Tables 2 and 3 ). The prevalence of C. difficile in community-onset cases did not differ from that in hospital-onset cases (18% vs 21%, respectively; P = .4), and the prevalence of norovirus was higher in community-onset cases (6% vs 1%; P = .03).
AGE cases were identified year-round ( Figure 3A and 3B ). Norovirus was detected every month, most commonly in November–January, reaching a peak prevalence of 16% in December. Rotavirus showed a distinct peak in April. Among bacterial pathogens, C. difficile was also detected year-round and reached a peak prevalence of 23% in October.

Seasonality of viral and bacterial pathogens among inpatient and outpatient cases, from July 2016 to June 2018. A, Viral pathogens. B, Bacterial pathogens. Abbreviations: C. difficile, Clostridioides difficile ; EIEC, enteroinvasive Escherichia coli .
Norovirus-positive samples were typed into 7 GI and 12 GII types; GII.4 Sydney[P16] (56%) viruses were most common, followed by GII.2[P16] (11%) ( Figure 4 ). The most common rotavirus genotypes were G12P[8] (79%) and G2P[4] (11%). For inpatient AGE cases with clinician-ordered testing data, 83% of C. difficile cases (55 of 66) positive by BioFire assay were also tested with PCR in the respective institution’s clinical laboratory (Cepheid Xpert C. difficile or Xpert C. difficile/Epi; Cepheid); of these cases, 87% (48 of 55) were also positive for C. difficile by clinical laboratory test. Clinical symptoms were similar among C. difficile –positive cases tested by both methods (data not shown).

Genotypes for norovirus (n = 71) and rotavirus (n = 19).
Incidence Estimates
The incidence of AGE hospitalizations was higher in adults ≥65 old (459 per 100 000 population) than in those <65 years old (315 per 100 000) ( Table 2 ). For outpatients, the reverse pattern was observed, with a slightly higher incidence in those aged <65 years (3100 vs 2247 per 100 000 in those aged ≥65 years). These age group and setting trends were also observed for norovirus, Campylobacter , and rotavirus. Of note, C. difficile and Salmonella had higher rates in adults ≥65 years of age for both inpatients and outpatients.
Incidence Rate Estimates Among Acute Gastroenteritis Case Patients (Inpatients and Outpatients), by Pathogen and Overall, July 2016 to June 2018
Abbreviations: AGE, acute gastroenteritis; CI, confidence interval; EIEC, enteroinvasive Escherichia coli.
a CIs for AGE outpatient estimates and inpatient community and hospital-onset inpatient estimates were calculated assuming Poisson distribution. Remaining estimates including pathogen-specific CIs calculated using bootstrapping.
b The attributable fraction (AF) was calculated for inpatient pathogens based on the odds ratio (OR) of that pathogen’s prevalence among case patients and controls, using the following formula: (OR –1)/OR. The AF is an estimate of the percentage of AGE cases that can be attributed to infection with the pathogen of interest. (The AF was not calculated for other inpatient variables, owing to the small sample size for controls after stratification.)
C. difficile and norovirus had the highest inpatient and outpatient incidence rates ( C. difficile, 71.3 and 285 per 100 000, respectively; norovirus, 19.4 and 291 per 100 000). The hospital-onset rate for C. difficile (114 per 100 000) was notably higher than that other pathogens, and higher than the C. difficile community-onset rate (55.6 per 100 000). For norovirus, the community-onset rate (18.3 per 100 000) was more than twice the hospital-onset rate (7.6 per 100 000). Norovirus incidence was twice as high in winter (November–April) as in summer (May–October), among both outpatients (542 vs 253 per 100 000) and inpatients (29 vs 12.5 per 100 000).
Clinical Symptoms and Severity of Illness Among Inpatients
Almost all inpatients with AGE had diarrhea (94%), and half had vomiting; the median duration of illness at follow-up was 7 days ( Table 3 ). By modified Vesikari clinical severity scores, inpatient AGE cases were largely classified as moderate (42%) or severe (52%). Among all pathogens, norovirus, rotavirus, and Shigella /EIEC had higher median severity scores, and the largest proportions of severe scores (73%, 73%, and 80%, respectively).
Clinical Symptoms and Severity of Illness for Inpatient AGE cases, Overall and by Single and Multiple Pathogens a
a Pathogen-specific columns include AGE cases with single pathogen detections, independent of the other 5 pathogens in the table. Any co-detection (≥2) between the six pathogens are included in the multiple co-pathogens column.
b If cases were missing data for certain measurements, a Vesikari score was not assigned, pathogen (% missing): AGE (4%), Norovirus (3%), C. difficile (6%), Salmonella (8%). Modified Vesikari scoring was defined as follows: Mild (0–6), Moderate (7–10), Severe (≥11). See supplementary appendix for scoring components.
c Among these deaths, 14 occurred while hospitalized. Three deaths occurred within 1–12 days following discharge; one died in inpatient hospice, one died within 24 hours of transferring to inpatient palliative care, and one died after discharge. Deaths following discharge were ascertained when attempting the follow-up interview at 3–5 weeks post-enrollment.
Campylobacter spp. includes jejuni, coli, and upsaliensis. EIEC= Enteroinvasive E. coli
d Diarrhea was defined as 3 or more loose stools within a 24 hour period
Overall, 12% of AGE case patients had ICU stays. By pathogen, Shigella /EIEC and C. difficile case patients had the highest proportion of ICU stays (20% and 17%, respectively) ( Table 3 ). By exposure, patients with hospital-onset AGE were twice as likely to have an ICU stay, and their median hospital stay was almost 4 times that of patients with community-onset AGE (22% vs 10% and 15 vs 4 days, respectively; both P < .01). Among the medical conditions examined (HIV, cancer, transplants, immunosuppressive therapy, and renal disease), only severe renal disease or renal failure was associated with more severe disease in patients with AGE; those with renal disease had a longer median hospital stay and a higher death rate than those without renal disease (7 vs 5 days [ P = .005] and 8% vs 2% [ P = .01], respectively).
Seventeen deaths (2%) were captured among inpatients with AGE. These included 1 norovirus-associated and 3 C. difficile –associated deaths. There was 1 other enteric detection among the deaths (enteroaggregative E. coli ), but based on the clinical course of illness and detailed record review, this pathogen was not believed to be a contributing factor. In the remaining 12 deaths, no pathogens were detected.
SUPERNOVA is a novel multisite AGE surveillance platform in the United States using prospective, active and passive, population-based surveillance with standardized case definitions and laboratory confirmation in a medically attended veteran population. We captured mild to severe AGE illness, including hospitalized cases, ICU stays, and deaths, and identified higher risk groups for AGE, including patients with certain medical conditions (HIV, transplants, and renal disease), and older adults. Testing for a wide range of AGE pathogens in a standardized fashion allowed us to identify C. difficile and norovirus as leading AGE pathogens, detected in inpatient and outpatient settings every month of the year, with a fall and winter predominance. These data will aid clinicians in clinical diagnosis and management for adults with AGE seeking medical care.
Norovirus was the leading viral pathogen in inpatients and outpatients with AGE. In our multisite surveillance, it is the second leading pathogen after C. difficile in inpatients with AGE, and the first in outpatients with AGE. Previous single-setting burden estimates for norovirus in US adults suggested that outpatient visits account for a greater proportion of cases than inpatient hospitalizations [ 3 , 9 , 15 , 17 ]. Our findings build on these studies, demonstrating higher norovirus prevalence in outpatients (10.7%) and inpatients with community-onset AGE (6%) than in those with hospital-onset AGE (1%), and norovirus incidence estimates >1 order of magnitude greater in outpatient (291 per 100 000) than in inpatient (19.4 per 100 000) and hospital-onset settings (7.6 per 100 000).
We also demonstrate that sporadic cases of norovirus were more common in the fall and winter months, from October through January, reaching peak prevalence of 1 in 6 AGE cases in December, and genotype GII.4 Sydney[P16] was detected in just more than half of norovirus positives. Although the genotypes are consistent with national outbreak surveillance [ 22 ], our outpatient and hospital surveillance detected an increase in norovirus cases approximately 2 months earlier in the fall. Earlier detection in the norovirus winter season can alert public health professionals to increase prevention and control efforts, and provide clinicians with timely data to inform appropriate testing and clinical decision making. We also demonstrate the heightened risk of older adults infected with norovirus. Every year in the United States, adults ≥65 years old account for almost half of norovirus hospitalizations and are at high risk for severe norovirus disease, including longer duration of symptoms and death [ 23 ]. In our study, norovirus prevalence was significantly higher in adults ≥85 years old, and the incidence of hospitalizations was almost twice as high in adults ≥65 years old as in younger adults.
C. difficile was the leading pathogen among inpatients; hospital-onset incidence was >10 times higher than any other pathogen, and twice the C. difficile community-onset incidence. Substantial C. difficile burden among veterans has been recognized [ 24 , 25 ], and a national longitudinal study of veterans in 2003–2014 found increasing rates for initial and recurrent C. difficile episodes over time [ 25 ]. However, retrospective studies relying on ICD codes and laboratory test results are influenced by changing diagnostics, including increased use of nucleic acid amplification tests, which are more sensitive than toxin assays. More recent data from Centers for Disease Control and Prevention demonstrate that although community-associated C. difficile cases have increased, healthcare-associated cases are decreasing [ 26 , 27 ]. In addition, there is increased recognition that many hospitalized patients with toxigenic C. difficile in their stool, identified either by toxigenic culture or nucleic acid amplification test (as in our surveillance), may be only colonized. This is reflected in clinical practice guidelines that highlight the importance of testing only patients with a high pretest likelihood for C. difficile and using more specific tests when this cannot be reasonably assured, as well as consideration of a multistep algorithm for testing [ 28 ]. Together with the high detection rate of C. difficile in non-AGE controls, this suggests that our C. difficile attributable fraction estimates, which indicate a 40% lower inpatient incidence rate, may provide more realistic estimates. From a clinical standpoint, these findings suggest that clinicians may consider the colonization rate in controls before starting antibiotic treatment in the setting of a positive result. Nonetheless, even with these lower adjusted estimates, C. difficile remained the leading inpatient pathogen, and adults ≥65 years of age with C. difficile had higher incidences than younger adults in both inpatients and outpatients, an important finding because older adults have higher morbidity and mortality rates related to C. difficile infection [ 29 ].
Our platform was also unique in its capture of adult rotavirus AGE cases, which demonstrated a distinct winter-spring peak and higher incidence in the first surveillance year. Rotavirus is a well-known cause of diarrhea in children, yet the epidemiology and burden of rotavirus in adult populations is not well understood, particularly in the postvaccination era. Indeed, we identified only 1 other study in the United States reporting rotavirus incidence rates in adults in the ambulatory setting, using an insurance claim database in the prevaccine era in outpatient and emergency rooms [ 9 ]. The reported incidence in the outpatient setting (8–19 per 100 000) was much lower than in our study (overall, 81 per 100 000; winter peak, 142 per 100 000); this difference could be due in part to infrequent stool diagnostic testing for rotavirus in adults, and it demonstrates the strength of our surveillance. Adult rotavirus prevalence estimates are similarly sparse. Interestingly, our rotavirus prevalence estimates (inpatients, 1.5%; outpatients, 3%) were similar to that reported from a hospital that retrospectively tested adult stool specimens collected for bacterial stool culture February to May each year from 2006 to 2011 (2.8%), as well as outpatient adult stool specimens submitted to a managed care organization in 2012–2013 (1%–3%) [ 30 , 31 ]. Widespread rotavirus vaccination in children has altered the US rotavirus seasonal pattern to biennial peaks from January to April [ 32 ], and G12P[8] has been the predominant strain detected since 2012 [ 21 ]; both the seasonality and genotyping in children are consistent with our findings in the current adult population.
The severity of disease for several pathogens was notable. Shigella /EIEC, norovirus, and rotavirus cases had the highest proportion of severe disease as measured by modified Vesikari score, whereas Shigella /EIEC and C. difficile cases had the highest proportion of ICU stays. Case patients with C. difficile had the longest median hospital stay, and those with norovirus the longest median ICU stay. Among AGE case patients who died, C. difficile and norovirus were the only pathogens detected that were believed to contribute to death. These patients had significant medical histories in addition to AGE, including cardiac, pulmonary, and renal disease that contributed to their hospitalization course and deaths.
In addition to specific pathogens, our case definition and data collection among all AGE case patients allowed for identification of subpopulations with AGE that may be at higher risk for more severe disease. First, inpatients ≥65 years old with AGE had higher disease incidence and longer median hospital stays than younger adults. Second, patients with AGE were more likely than controls to have HIV, be transplant recipients, or have severe or end-stage renal disease. In particular, AGE case patients with severe renal disease or renal failure had a longer median hospital stay and a death rate that was 4 times higher than AGE case patients without renal disease. Although diarrhea is common in patients with end-stage renal disease, its etiology can be challenging to determine and may include both infectious and noninfectious causes [ 33 ]; as such, this finding merits additional investigation as our surveillance continues. A third group of patients, with hospital-onset AGE, while representing only 1 in 5 AGE hospitalizations, were twice as likely to have an ICU stay and were notable for their higher AGE incidence compared with community-onset case patients. Patients with hospital-onset AGE had longer stays, almost 4 times longer than in patients with community-onset AGE.
Our findings are subject to limitations. First, it was challenging to obtain consent and enroll ICU patients with AGE, and some patients with AGE died before enrollment; therefore, our surveillance may underestimate AGE severity. As previously noted, PCR-based assays are sensitive, and bacterial pathogens were not independently confirmed by culture or toxin assay; detection may not indicate causation. However, the significantly higher case detection rates compared with controls, high single-pathogen detection among C. difficile and norovirus cases, and clinical symptoms and severity consistent with expected presentations for these pathogens gives us confidence in our interpretation. Finally, veterans are predominantly male, and results may not be representative of all US adults.
In conclusion, we offer robust assessment of the burden of AGE and specific AGE pathogens, including norovirus and C. difficile , in an adult population before vaccine introduction for these pathogens. Ongoing surveillance will allow further characterization of AGE pathogens, genotyping, burden, and risk factors in adults. Clinicians can use these data to inform clinical diagnosis and management for adults seeking medical care for AGE.
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. The authors thank the study site participants for their time and contributions. They also gratefully acknowledge the following individuals for their contributions to the surveillance system and laboratory testing: Emory University: Ben Lopman, Jessica Ingersoll, and Candace Miller; Baylor College of Medicine: Robert Atmar and Frederick Neill; Houston Veterans Affairs Medical Center (VAMC): Mahwish Mushtaq, David Diaz Voss Varela, Rosalba Gomez, Bashir Lengi, Ernesto Ruiz, and Martha Bilbatua; Atlanta VAMC: Nana Addo Padi-Adjirackor, Elena Morales, Janet Thonkulpitak, Theron Clark-Stuart, Abeer Moanna, and Nora Oliver; Bronx VAMC: Johane Simelane, Sarah Smith, Amelia Tisi, and Guerry (Anabelle) Perez; Los Angeles VAMC: Matthew Goetz, Anthony Matolek, Evan Goldblatt, and Aleksandra (Sasha) Poteshkina; and Palo Alto VAMC: Madhuri Agrawal, Jessica Lopez, and Jude Lopez.
Disclaimer. The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government.
Financial support. This study was funded by the Centers for Disease Control and Prevention and by the Emory Center for AIDS Research (funding for laboratory personnel at the Atlanta VAMC).
Potential conflicts of interest. V. C. M. reports grants from Gilead, ViiV, and Bayer, and personal fees from Lilly, Gilead, and ViiV, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
GBD Diarrhoeal Diseases Collaborators . Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015 . Lancet Infect Dis 2017 ; 17 : 909 – 48 .
Scallan E , Griffin PM , Angulo FJ , Tauxe RV , Hoekstra RM . Foodborne illness acquired in the United States—unspecified agents . Emerg Infect Dis 2011 ; 17 : 16 – 22 .
Google Scholar
Lopman BA , Hall AJ , Curns AT , Parashar UD . Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996-2007 . Clin Infect Dis 2011 ; 52 : 466 – 74 .
Hall AJ , Curns AT , McDonald LC , Parashar UD , Lopman BA . The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007 . Clin Infect Dis 2012 ; 55 : 216 – 23 .
Hall AJ , Lopman BA , Payne DC , et al. Norovirus disease in the United States . Emerg Infect Dis 2013 ; 19 : 1198 – 205 .
Lessa FC , Mu Y , Bamberg WM , et al. Burden of Clostridium difficile infection in the United States . N Engl J Med 2015 ; 372 : 825 – 34 .
Payne DC , Vinjé J , Szilagyi PG , et al. Norovirus and medically attended gastroenteritis in U.S. children . N Engl J Med 2013 ; 368 : 1121 – 30 .
Centers for Disease Control and Prevention . Clostridioides difficile infection (CDI) tracking. Available at: https://www.cdc.gov/hai/eip/cdiff-tracking.html . Accessed 7 January 2020 .
Gastañaduy PA , Hall AJ , Curns AT , Parashar UD , Lopman BA . Burden of norovirus gastroenteritis in the ambulatory setting—United States, 2001-2009 . J Infect Dis 2013 ; 207 : 1058 – 65 .
Hall AJ , Rosenthal M , Gregoricus N , et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005 . Emerg Infect Dis 2011 ; 17 : 1381 – 8 .
Vinjé J . Advances in laboratory methods for detection and typing of norovirus . J Clin Microbiol 2015 ; 53 : 373 – 81 .
Cardemil CV , O’Leary ST , Beaty BL , et al. 1624. Primary care physician knowledge, attitudes, and diagnostic testing practices for norovirus and acute gastroenteritis . Open Forum Infect Dis 2019 ; 6 : S592 – 3 .
Mattison CP , Cardemil CV , Hall AJ . Progress on norovirus vaccine research: public health considerations and future directions . Expert Rev Vaccines 2018 ; 17 : 773 – 84 .
Riley TV , Lyras D , Douce GR . Status of vaccine research and development for Clostridium difficile . Vaccine 2019 ; 37 : 7300 – 6 .
Grytdal S , Browne H , Collins N , et al. Trends in incidence of norovirus-associated acute gastroenteritis in 4 Veterans Affairs Medical Center populations in the United States, 2011–2015 . Clin Infect Dis 2020 ; 70 : 40 – 8 .
Grytdal SP , Rimland D , Hannah Shirley S , et al. Incidence of medically-attended norovirus-associated acute gastroenteritis in four Veteran’s Affairs Medical Center populations in the United States, 2011–2012 . PLoS One 2015 ; 10 : e0126733 .
Kambhampati A , Vargas B , Mushtaq M , et al. Active surveillance for norovirus in a US Veterans Affairs patient population, Houston, Texas, 2015–2016 . OFID 2019 ; 6 : ofz115 .
Cannon JL , Barclay L , Collins NR , et al. Genetic and epidemiologic trends of norovirus outbreaks in the United States from 2013 to 2016 demonstrated emergence of novel GII.4 recombinant viruses . J Clin Microbiol 2017 ; 55 : 2208 – 21 .
Chhabra P , Payne DC , Szilagyi PG , et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008-2009 . J Infect Dis 2013 ; 208 : 790 – 800 .
Gautam R , Mijatovic-Rustempasic S , Esona MD , Tam KI , Quaye O , Bowen MD . One-step multiplex real-time RT-PCR assay for detecting and genotyping wild-type group A rotavirus strains and vaccine strains (Rotarix® and RotaTeq®) in stool samples . PeerJ 2016 ; 4 : e1560 .
Bowen MD , Mijatovic-Rustempasic S , Esona MD , et al. Rotavirus strain trends during the postlicensure vaccine era: United States, 2008–2013 . J Infect Dis 2016 ; 214 : 732 – 8 .
Wikswo ME , Kambhampati A , Shioda K , Walsh KA , Bowen A , Hall AJ ; Centers for Disease Control and Prevention . Outbreaks of acute gastroenteritis transmitted by person-to-person contact, environmental contamination, and unknown modes of transmission—United States, 2009–2013 . MMWR Surveill Summ 2015 ; 64 : 1 – 16 .
Cardemil CV , Parashar UD , Hall AJ . Norovirus infection in older adults: epidemiology, risk factors, and opportunities for prevention and control . Infect Dis Clin North Am 2017 ; 31 : 839 – 70 .
Appaneal HJ , Caffrey AR , Beganovic M , Avramovic S , LaPlante KL . Predictors of mortality among a national cohort of veterans with recurrent Clostridium difficile infection . Open Forum Infect Dis 2018 ; 5 : ofy175 .
Reveles KR , Lawson KA , Mortensen EM , et al. National epidemiology of initial and recurrent Clostridium difficile infection in the Veterans Health Administration from 2003 to 2014 . PLoS One 2017 ; 12 : e0189227 .
Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2019 . Atlanta, GA : US Department of Health and Human Services, Centers for Disease Control and Prevention , 2019 .
Google Preview
Centers for Disease Control and Prevention . Antibiotic resistance and patient safety portal. Available at: https://arpsp.cdc.gov/profile/infections/CDI . Accessed 7 January 2020 .
McDonald LC , Gerding DN , Johnson S , et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) . Clin Infect Dis 2018 ; 66 : 987 – 94 .
Jump RL . Clostridium difficile infection in older adults . Aging health 2013 ; 9 : 403 – 14 .
Anderson EJ , Shippee DB , Tate JE , et al. Clinical characteristics and genotypes of rotavirus in adults . J Infect 2015 ; 70 : 683 – 7 .
Grytdal SP , DeBess E , Lee LE , et al. Incidence of norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among Kaiser Permanente member populations in the United States, 2012–2013 . PLoS One 2016 ; 11 : e0148395 .
Aliabadi N , Haynes A , Tate J , Parashar UD , Curns AT . Trends in the burden and seasonality of rotavirus in the United States, 2000–2016 . Open Forum Infect Dis 2017 ; 4 : S324 .
Higashihara T , Okada A , Kishida Y , et al. Atypical cause of intractable diarrhea in a hemodialysis patient, masked by Clostridium difficile -associated diarrhea and ischemic colitis: a case report . BMC Nephrol 2018 ; 19 : 303 .
- gastroenteritis, acute
- hospitals, veterans
- intensive care unit
- outpatients
- pathogenic organism
- surveillance, medical
- enteroinvasive escherichia coli
- clostridium difficile
- stool specimen
Supplementary data
Email alerts, more on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- X (formerly Twitter)
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
Dr. John A. Weems (Medicine): A 29-year-old woman was evaluated at a primary care clinic affiliated with this hospital because of nausea, vomiting, and diarrhea.
ABOUT DISEASE ACUTE GASTROENTERITIS: Acute gastritis is a sudden inflammation or swelling in the lining of the stomach. It can cause severe and nagging pain. However, the pain is temporary and usually lasts for short bursts at a time.
The admitting diagnosis was Acute Gastroenteritis with Moderate to Severe Dehydration and developed into Sepsis later on. The student nurses aim to exhibit understanding regarding the disease, its risk factors and complications and its preventable measures.
He describes the quality of the abdominal pain as sharp, originating in the epigastric region and radiating to his back, and exacerbated by movement. Additionally, he has had several episodes of nonbloody, nonbilious vomiting and watery diarrhea.
Acute gastroenteritis is a common infectious disease syndrome, causing a combination of nausea, vomiting, diarrhea, and abdominal pain. There are more than 350 million cases of acute gastroenteritis in the United States annually and 48 million of these cases are caused by foodborne bacteria.
Infants below the age of 12 months are especially at an increased risk of morbidity and mortality. Our case is a 4-month-old male who presents with gastroenteritis in the ED and evaluated for sepsis. Stool cultures were taken and resulted in a positive salmonella gastroenteritis diagnosis. Gastroenteritis
Acute gastroenteritis (AG) is a common disease in humans worldwide. Case definition varies between studies and countries but mostly includes signs and symptoms of diarrhoea, vomiting, nausea, abdominal cramps or pain, fever, and blood or mucus in the stool [1–5].
A 2-month-old boy with acute Gastroenteritis admitted to Shahid Beheshti Hospital of Kashan, Iran. There were vomiting, alternating fever, and yellow-watery feces in historical and clinical examinations in 2-days ago.
Box 1 lists some causes of acute gastroenteritis in children. Worldwide, most cases are due to viral infection (fig 1; box 2), with rotaviruses and noroviruses being most common. Viral infections damage small bowel enterocytes and cause low grade fever and watery diarrhoea without blood.
Acute gastroenteritis (AGE), characterized by diarrhea, vomiting, fever, and abdominal pain, causes 1.3 million deaths globally every year . In the United States, AGE causes 179 million cases and 1 million hospitalizations annually [2, 3].